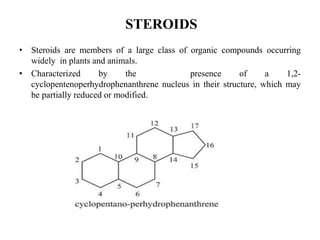
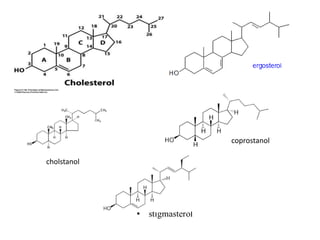
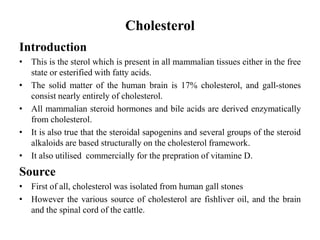
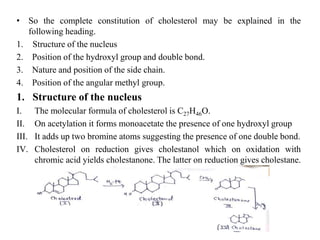
This document provides information about sterols and cholesterol. It defines sterols as crystalline steroids that contain an alcoholic group. Cholesterol is introduced as a key animal sterol present in mammalian tissues. The complete constitution of cholesterol is then elucidated, including that it has a tetracyclic nucleus containing a hydroxyl group at position 3 and a double bond between carbons 5 and 6, with an isohexylmethyl side chain attached at carbon 17. Several reactions are described to support the structural analysis of cholesterol.



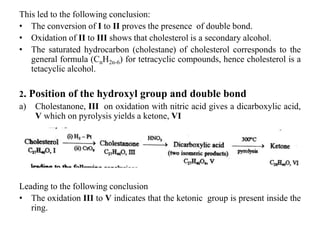
![• The conversation of dicarboxylic acid, V to a ketone, VI indicates that V is
either a 1, 6 or 1, 7- decarboxylic acid [blanc rule]. Now we see that this
dicarboxylic acid is obtained from the hydroxyl group of cholesterol which
can’t present in ring D as it would form a ,5- decarboxylic acid instead of 1,6-
or 1,7- decarboxylic acid on the above treatment. Hence the hydroxyl group
may be either in ring A,B or C.
• The formation of two isomeric dicarboxylic acids, V, suggest that the keto
group in cholestanone is flanked by a methylene group on either side(-
CH2.CO.CH2-) .
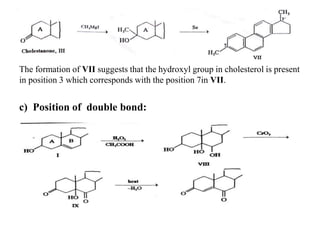
b) Cholestone, III. on treatment with methyl magnesium iodide followed by
selinium dehydrogenation yields 3’, 7- dimethylcyclopentenophenanthrene, VII,
the structure of which is proved by its synthesis.](https://image.slidesharecdn.com/cholesterol-201111103947/85/Cholesterol-9-320.jpg)