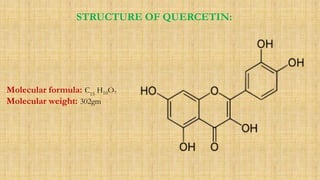
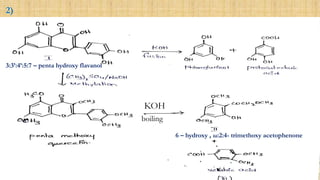
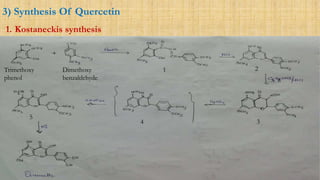
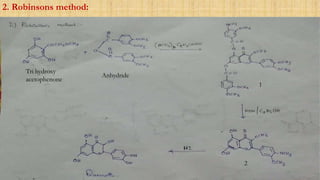
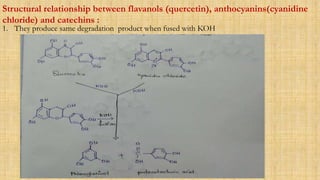
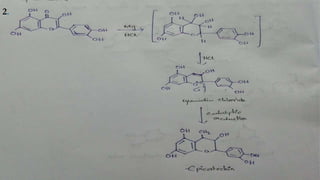
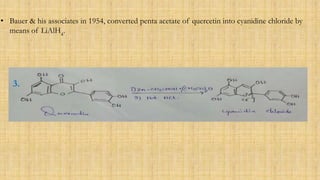
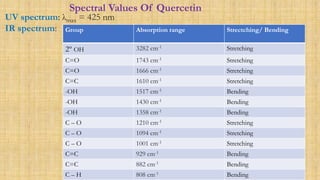
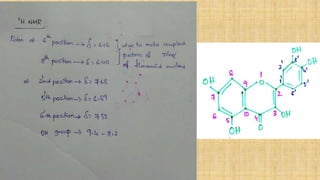
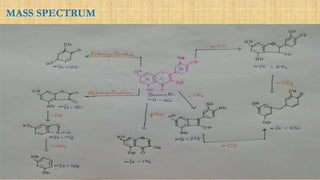
The document discusses the structural elucidation of quercetin, detailing its sources, molecular structure, and characteristics. It highlights the results of various spectral analyses that confirm the presence of hydroxyl, carbonyl, and aromatic groups, and elaborates on the synthesis methods of quercetin. The conclusion asserts that quercetin's structure is validated through comprehensive spectral data.