This document discusses glycosides, which are organic compounds derived from the replacement of a hydrogen atom of a cyclic hemiacetal by an organic radical. Glycosides are obtained from plant or animal sources and contain a sugar moiety (glycone) and non-sugar moiety (aglycone or genin). Important examples discussed include digitoxin, a cardiac glycoside used to treat congestive heart failure, sennoside which is a laxative anthraquinone glycoside, and diosgenin which is a steroid sapogenin extracted from yams and used to synthesize progesterone and cortisone. The document also covers classification, structures, properties and tests for various types of glycosides



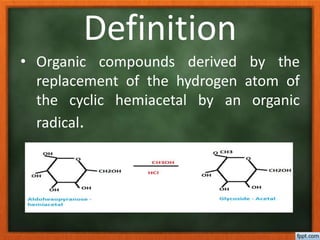
![• The hydroxyl group that is derived
from carbonyl group by ring
formation [C1 in aldoses and C2 in
ketoses] is involved in glycoside
formation this hydroxyl group is
known as Glycosidic hydroxyl group.
• The organic radical linked at
glycosidic hydroxyl group is acid
labile.](https://image.slidesharecdn.com/glycosides-180305103351/85/Glycosides-5-320.jpg)






![4.Cyanogenic glycoside
• A glycone contain a cyanide group.
• The glycoside can release the poisonous
hydrogen cyanide. Eg. Amygdalin from
almond.
• Cyanogenic glycosides can be found in
fruits [ includes cherries, apples, plums ,
almonds , peaches , apricots and
raspberries ]](https://image.slidesharecdn.com/glycosides-180305103351/85/Glycosides-14-320.jpg)

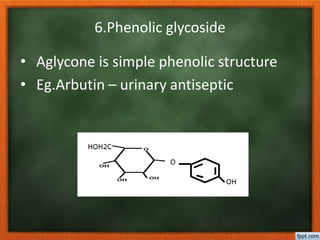

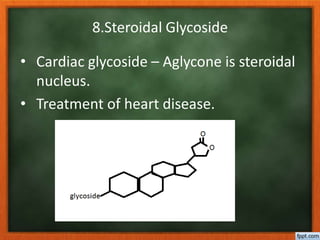




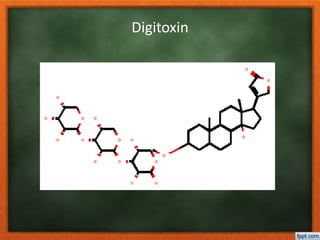





![Plant origin have cardenolide, possess α,β
unsaturated lactone ring varies with
source of glycoside.
Animals origin have bufadienolide possess
six membered pyranone ring with two
conjugated double bonds [α pyranone ]
O
O
O
O](https://image.slidesharecdn.com/glycosides-180305103351/85/Glycosides-30-320.jpg)





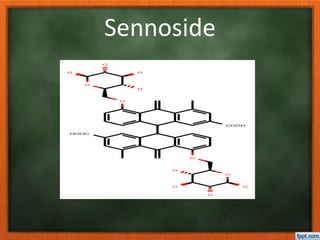

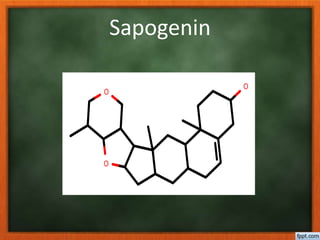
![Sapogenin
• The saponins [ latin sapo- Soap ] are
glycosides which lowers the surface
tension of water.
• The genin portion may be
pentacyclic triterpene C30
sapogenin or a c27 steroidal
sapogenin with a spiroketal side
chain [ spirostane ]](https://image.slidesharecdn.com/glycosides-180305103351/85/Glycosides-41-320.jpg)

