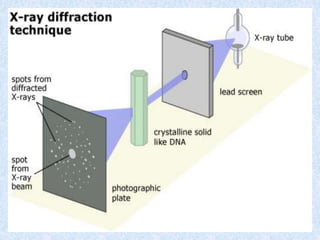
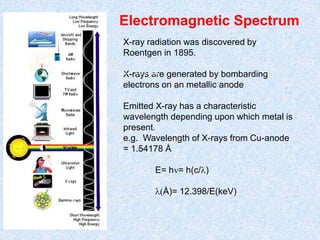
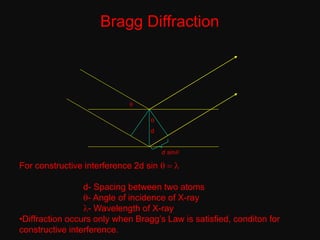
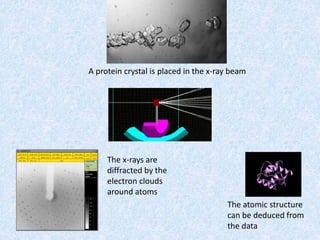
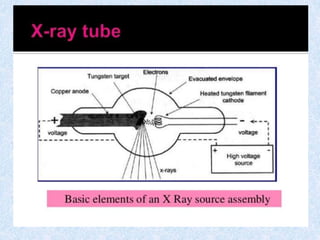
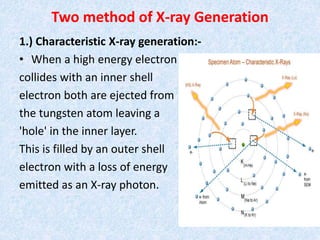
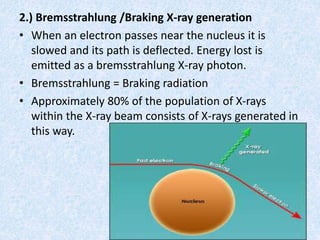
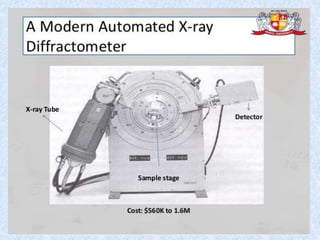
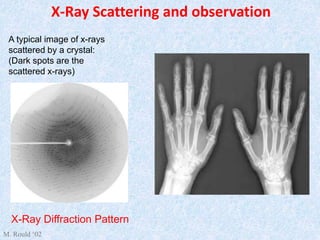
The document discusses X-ray diffraction, a non-destructive method of chemical analysis that produces unique diffraction patterns for crystalline substances, akin to a fingerprint. It highlights the principles of X-ray generation, methods of producing X-rays, and the advantages and disadvantages of X-ray techniques for studying atomic and molecular structures. Additionally, it covers specimen preparation and the significance of adherence to Bragg's law for constructive interference in diffraction observations.