The document discusses key concepts in thermodynamics and thermochemistry including:
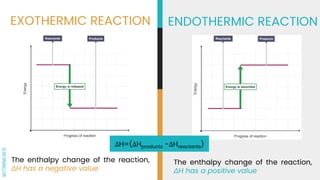
1) Thermochemistry is the study of energy changes that occur during chemical reactions. Reactions can be exothermic, releasing heat from the system, or endothermic, absorbing heat into the system.
2) The enthalpy change (ΔH) of a reaction indicates whether it is exothermic (negative ΔH) or endothermic (positive ΔH).
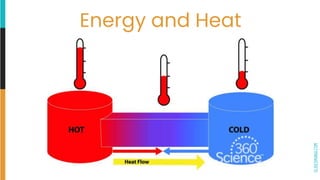
3) A system exchanges energy with its surroundings during chemical or physical changes. Exothermic reactions increase the temperature of the surroundings while endothermic reactions decrease it.