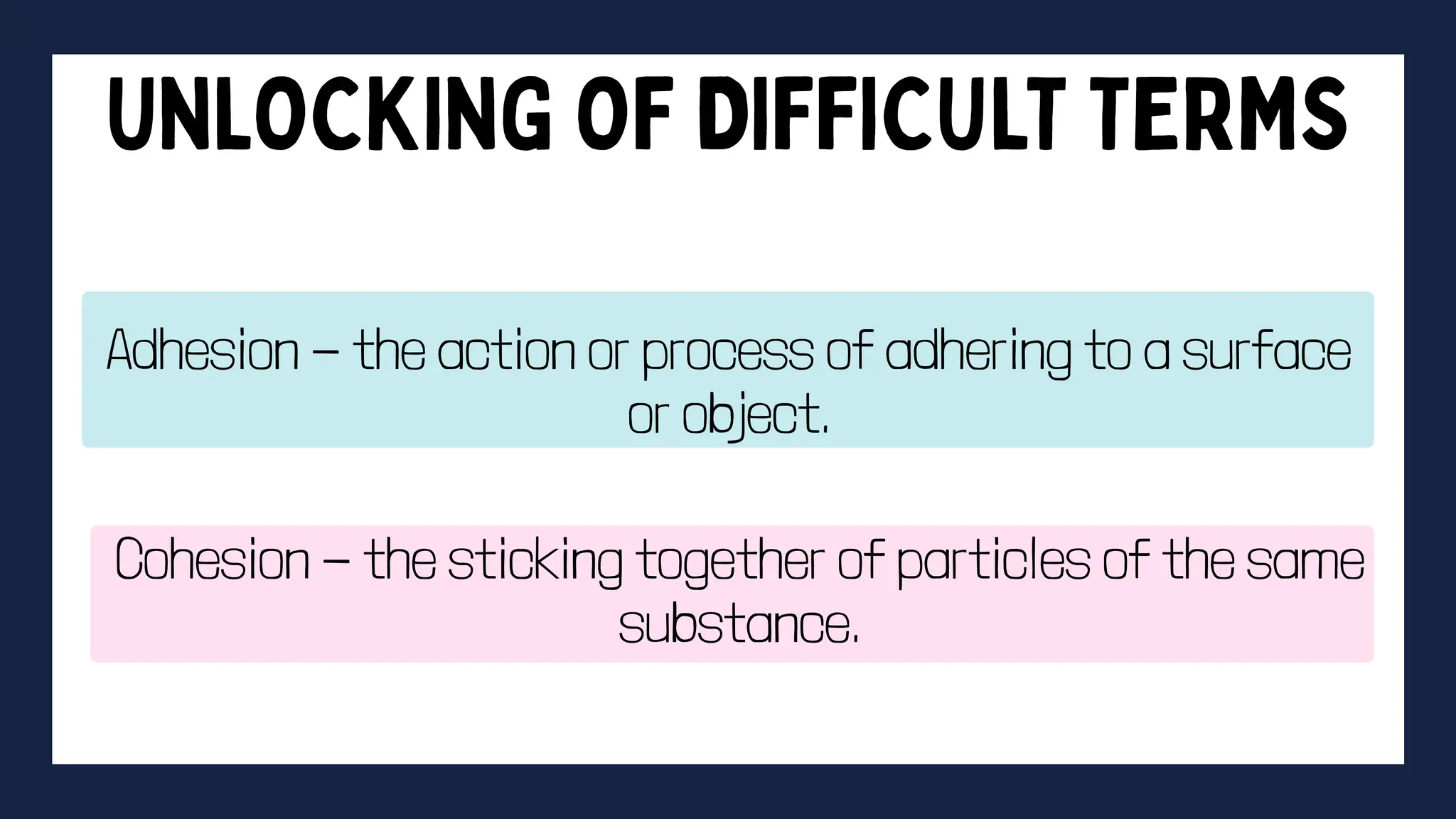
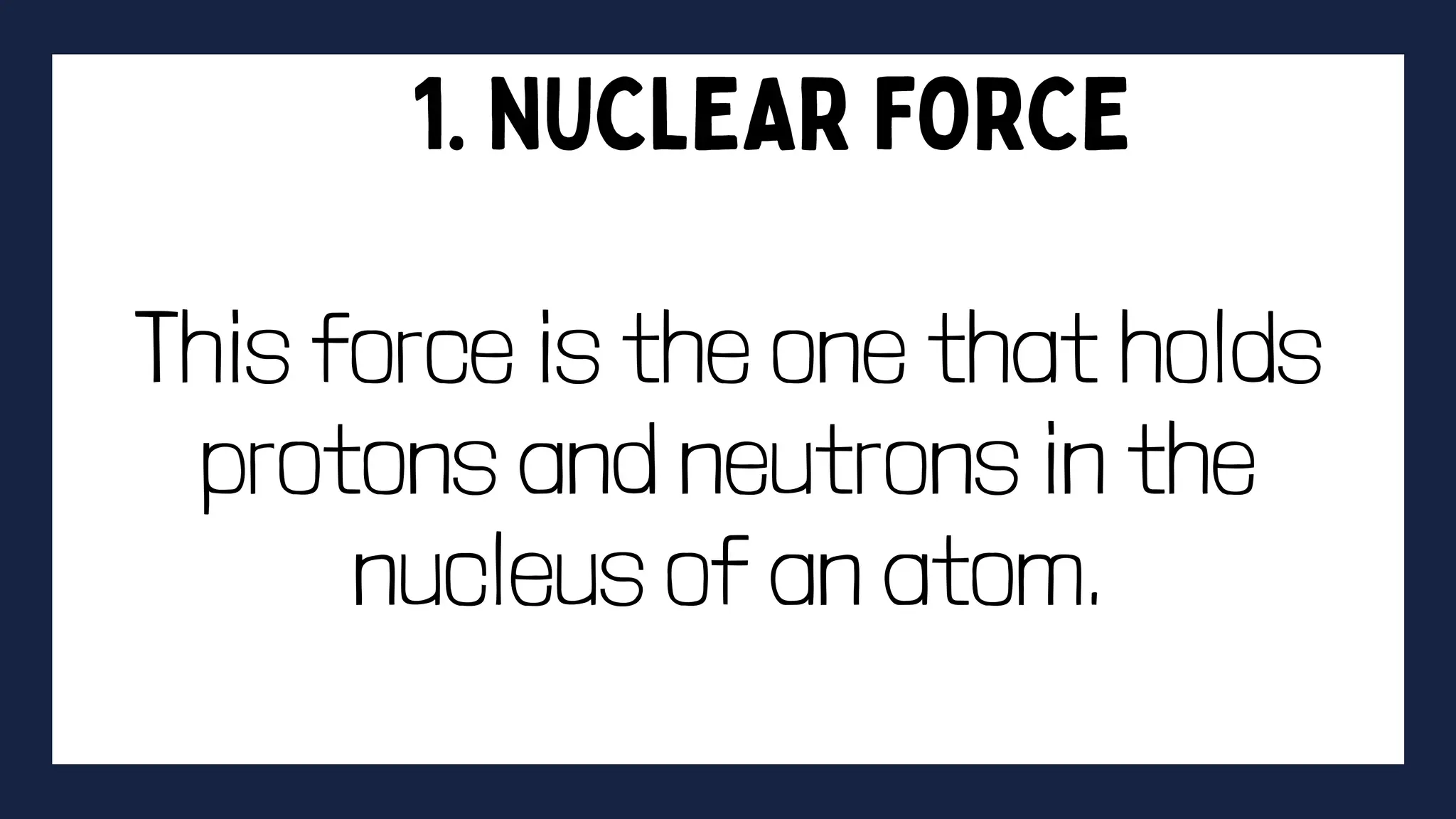
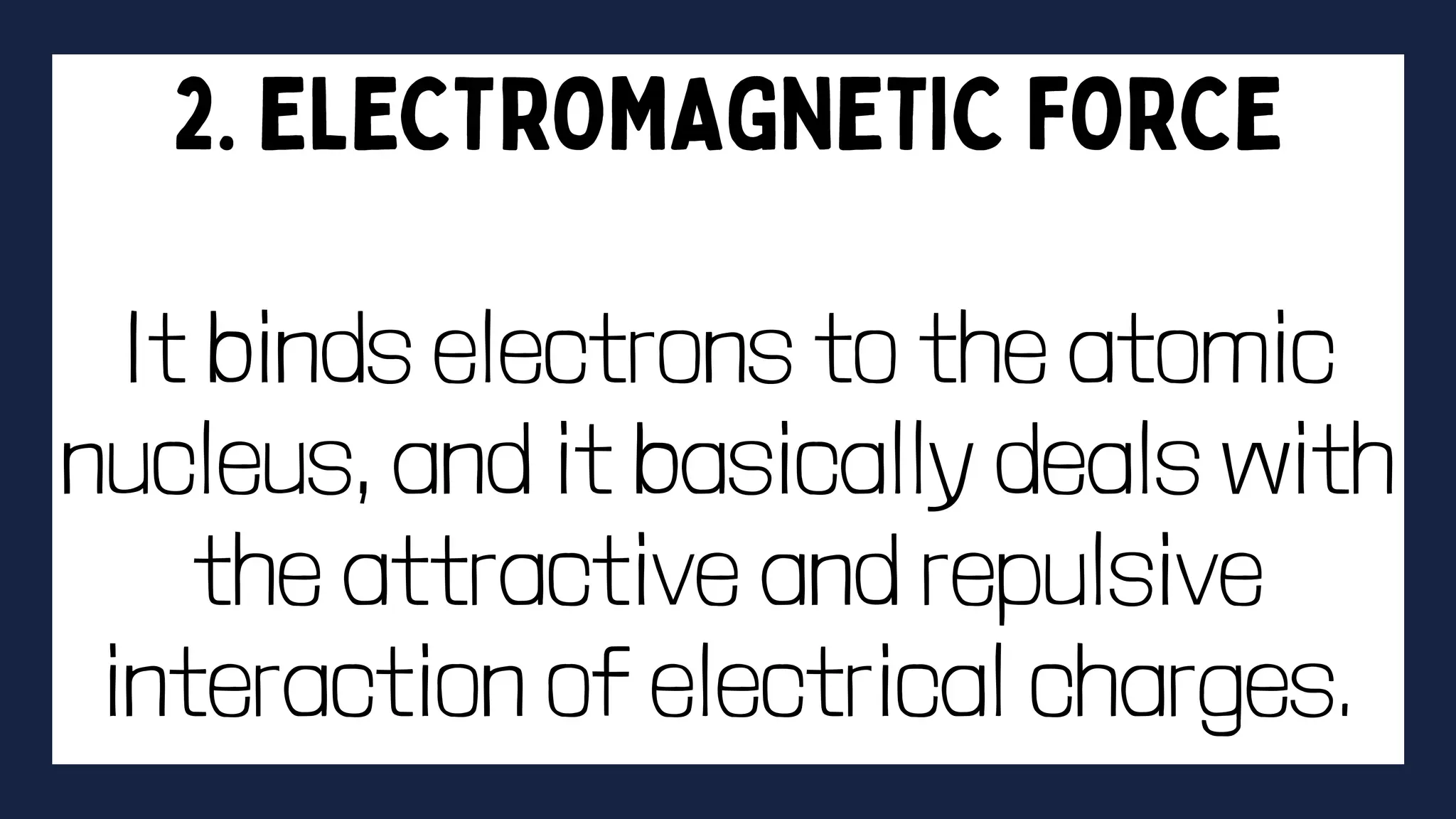
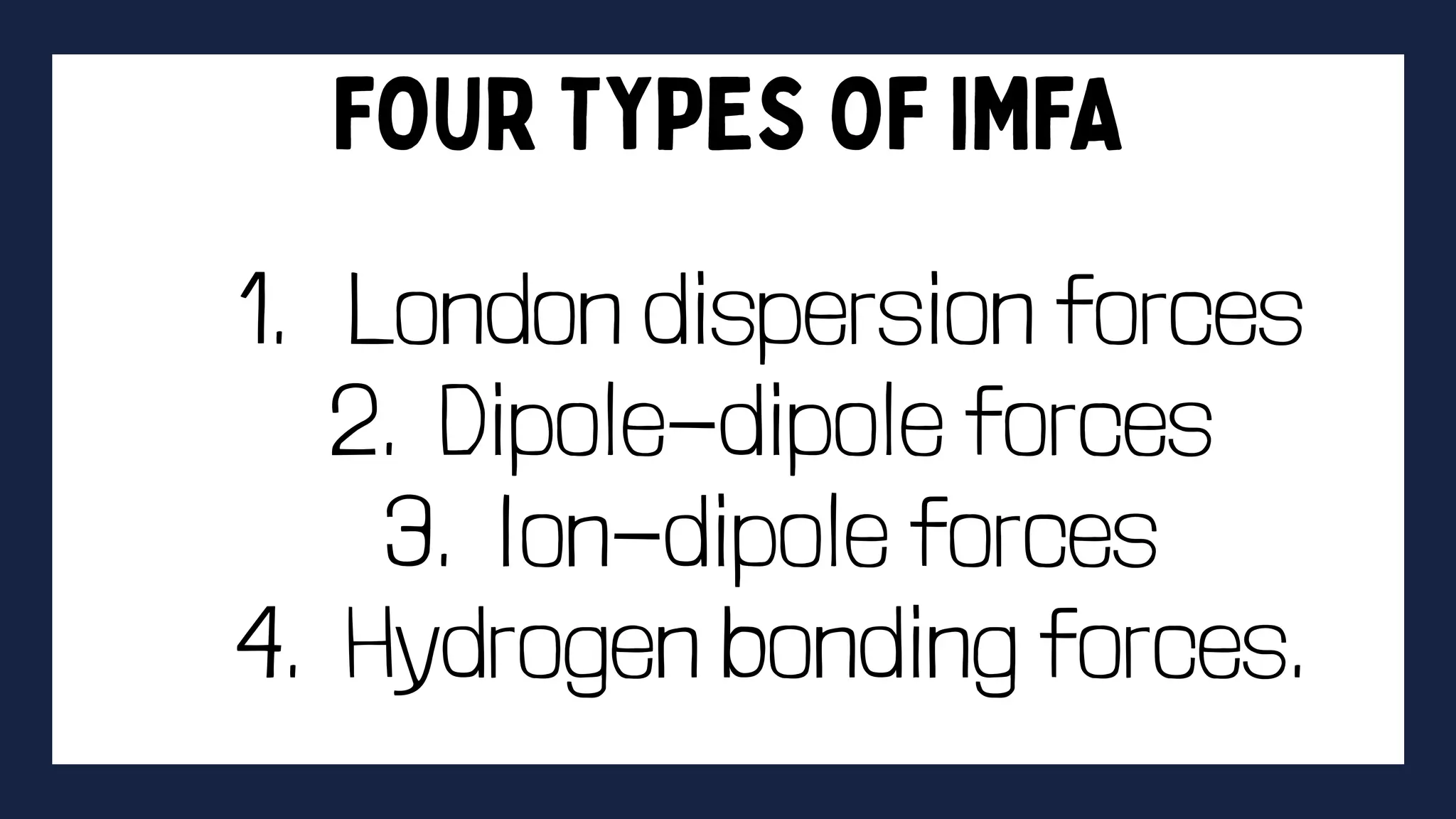
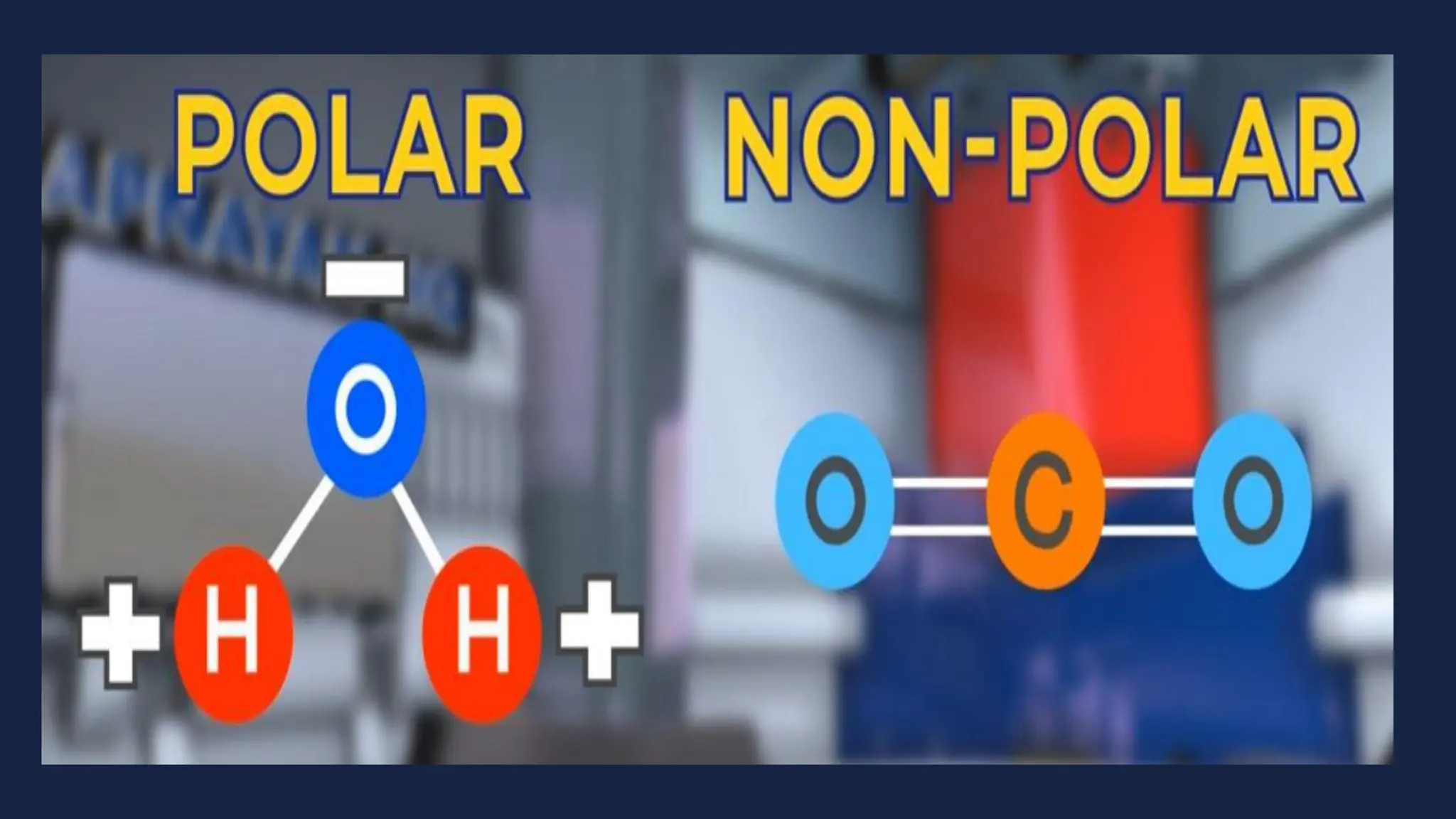
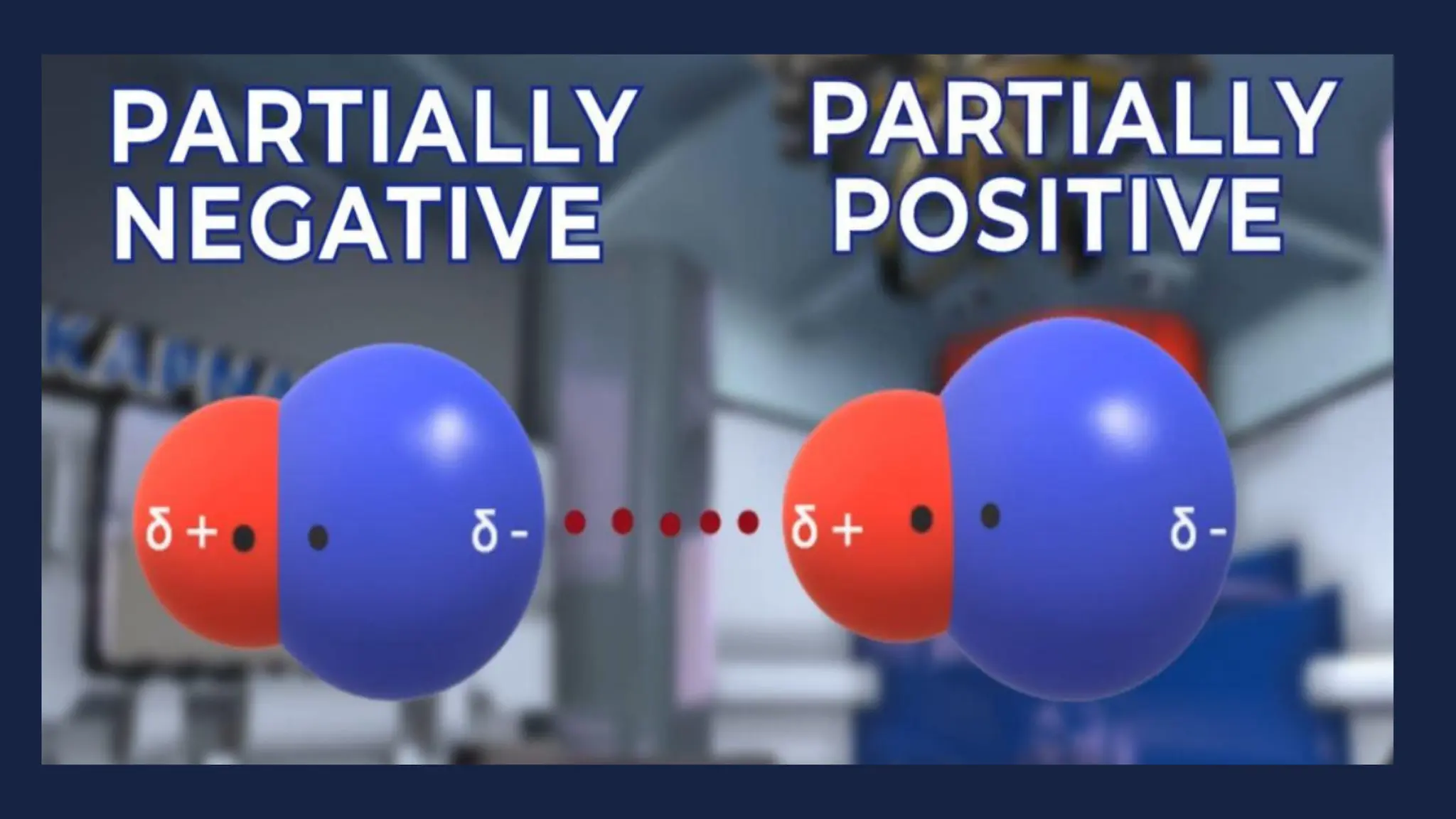
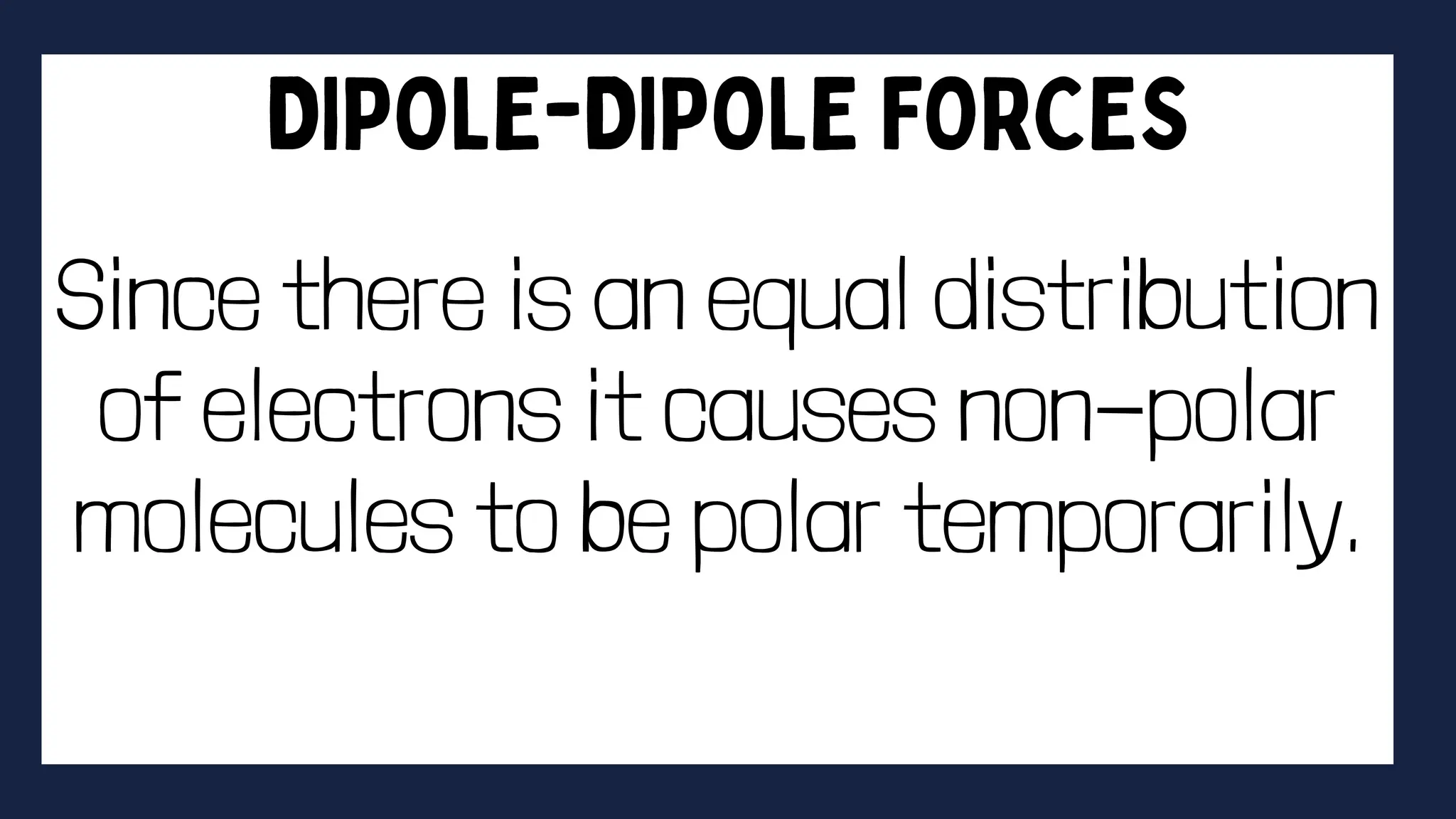
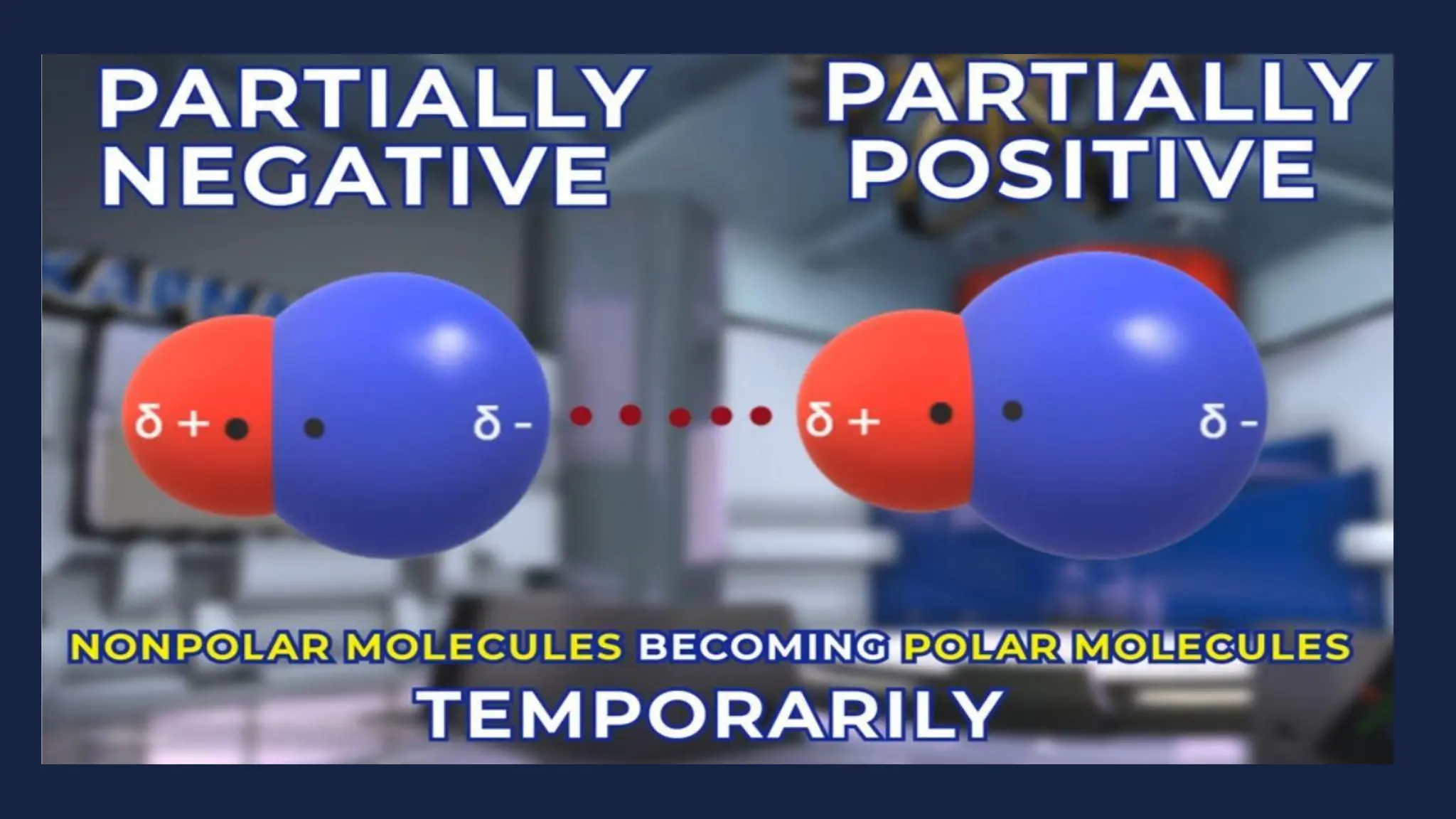
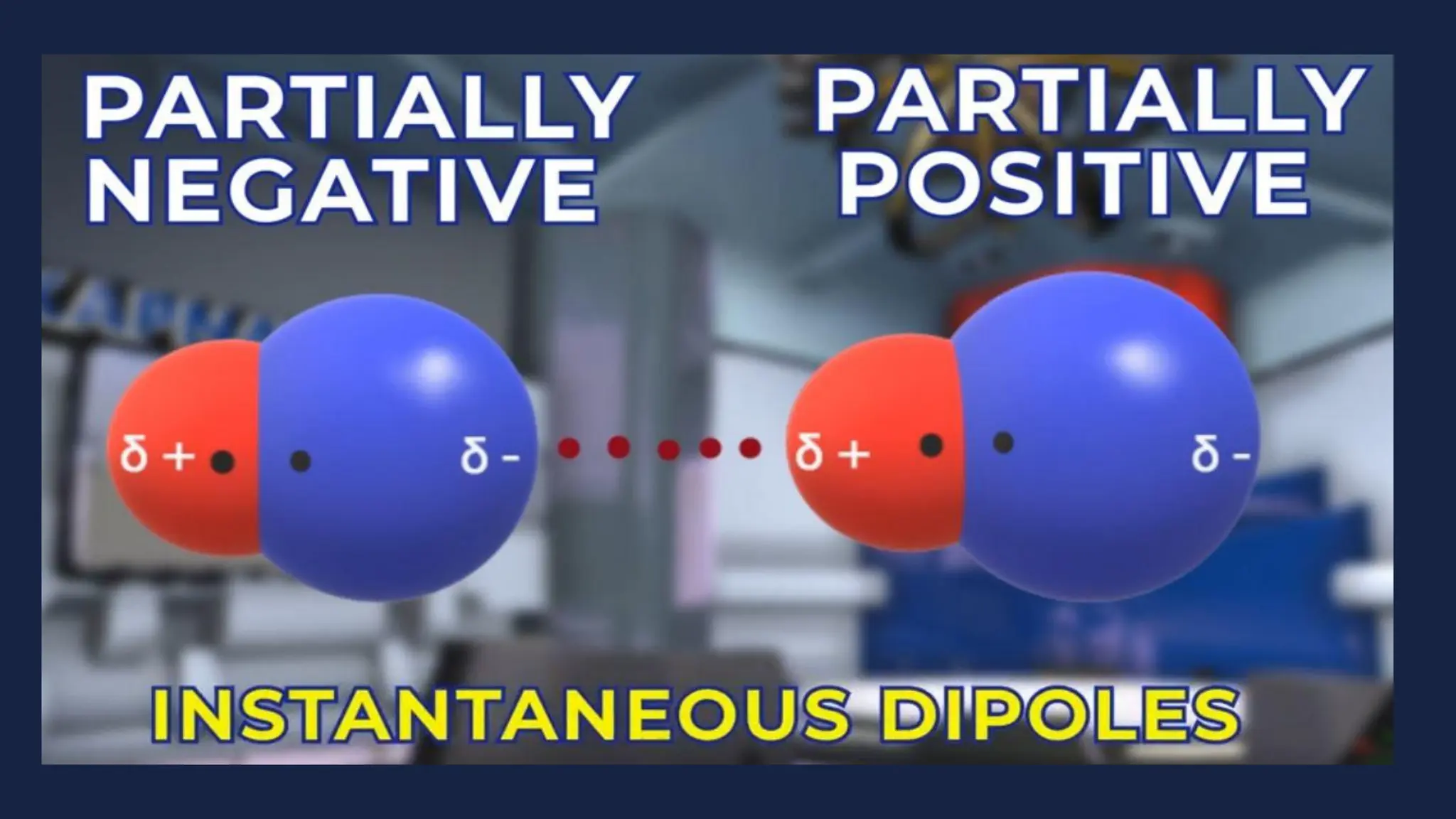
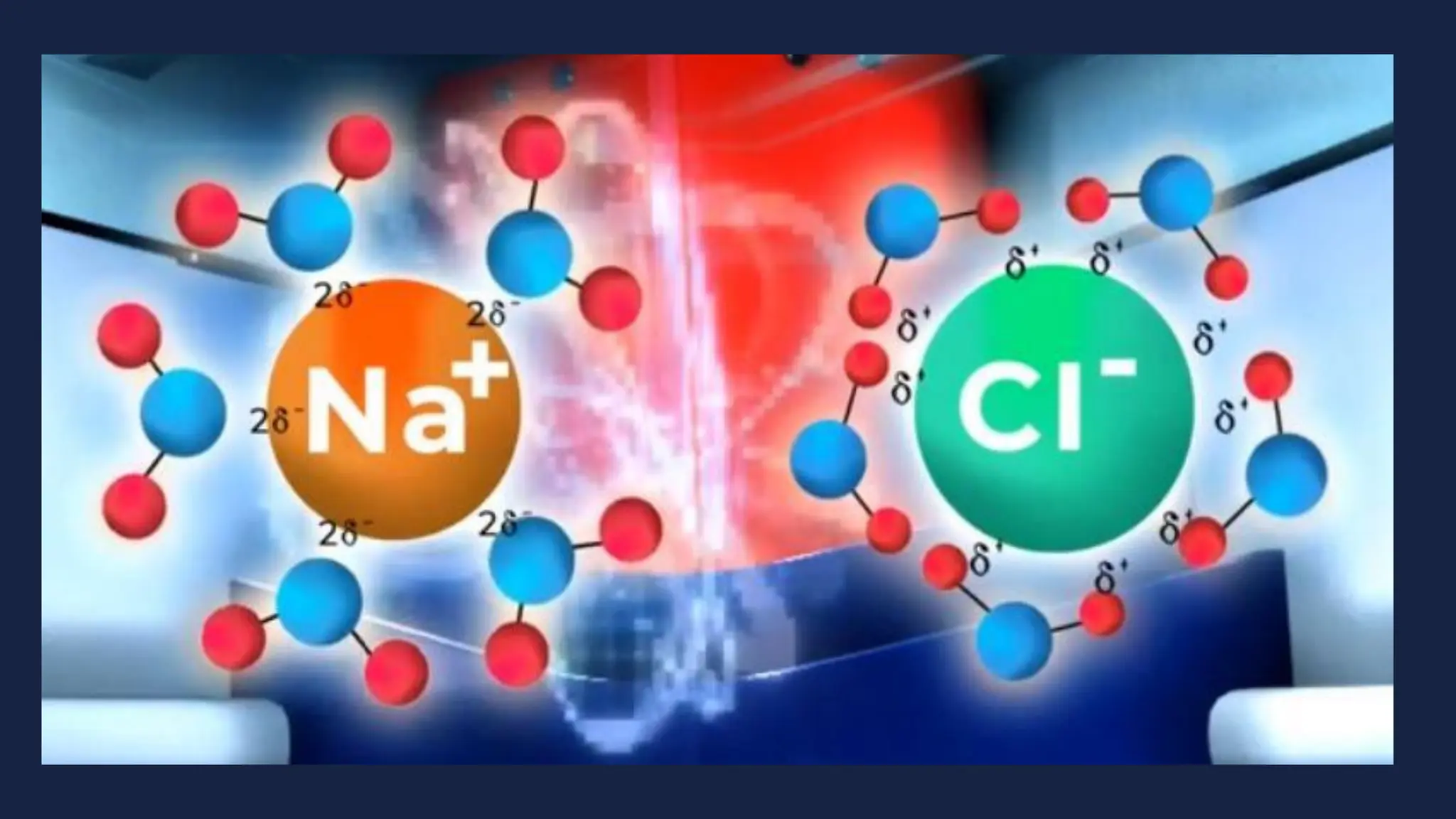
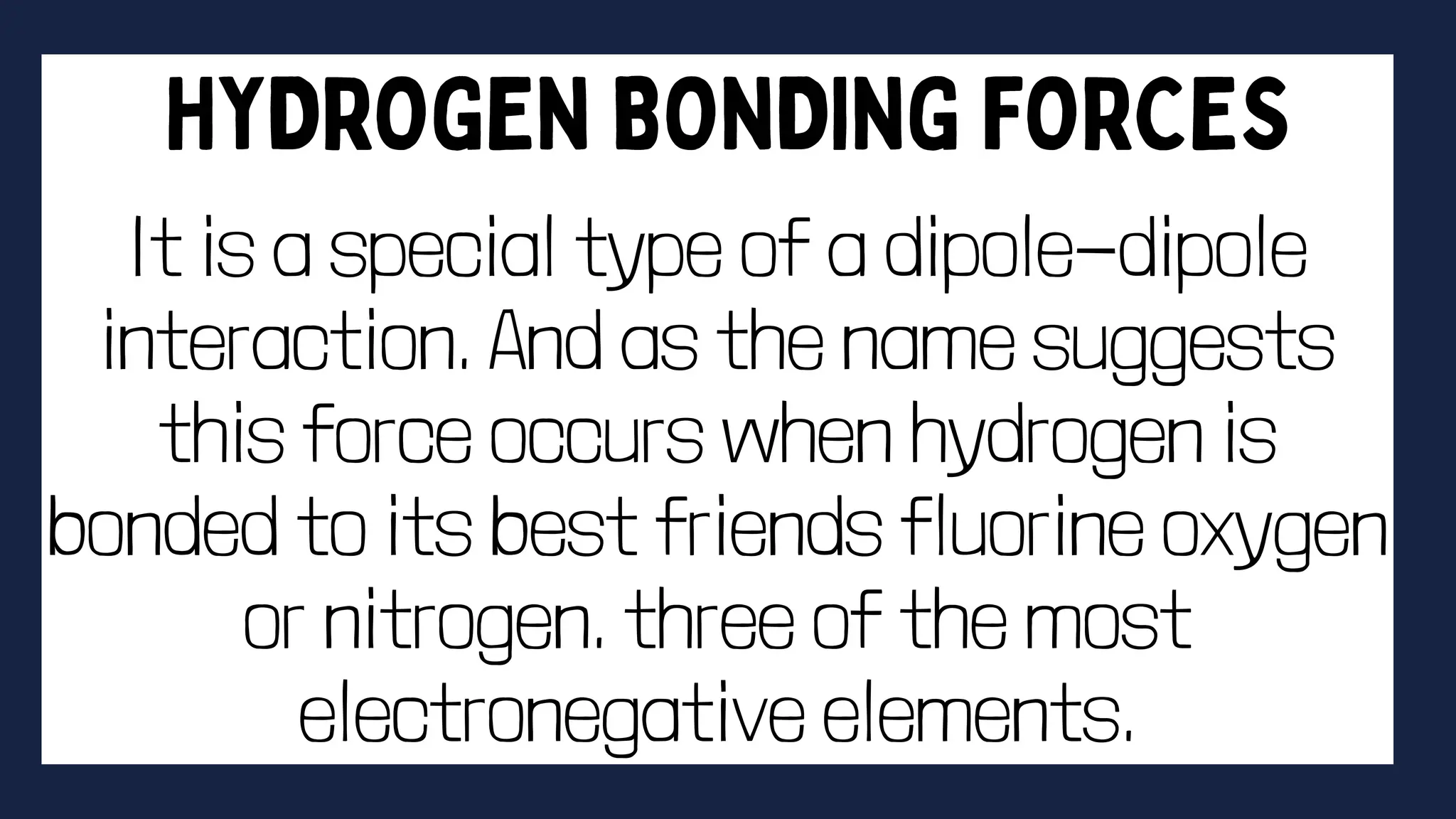
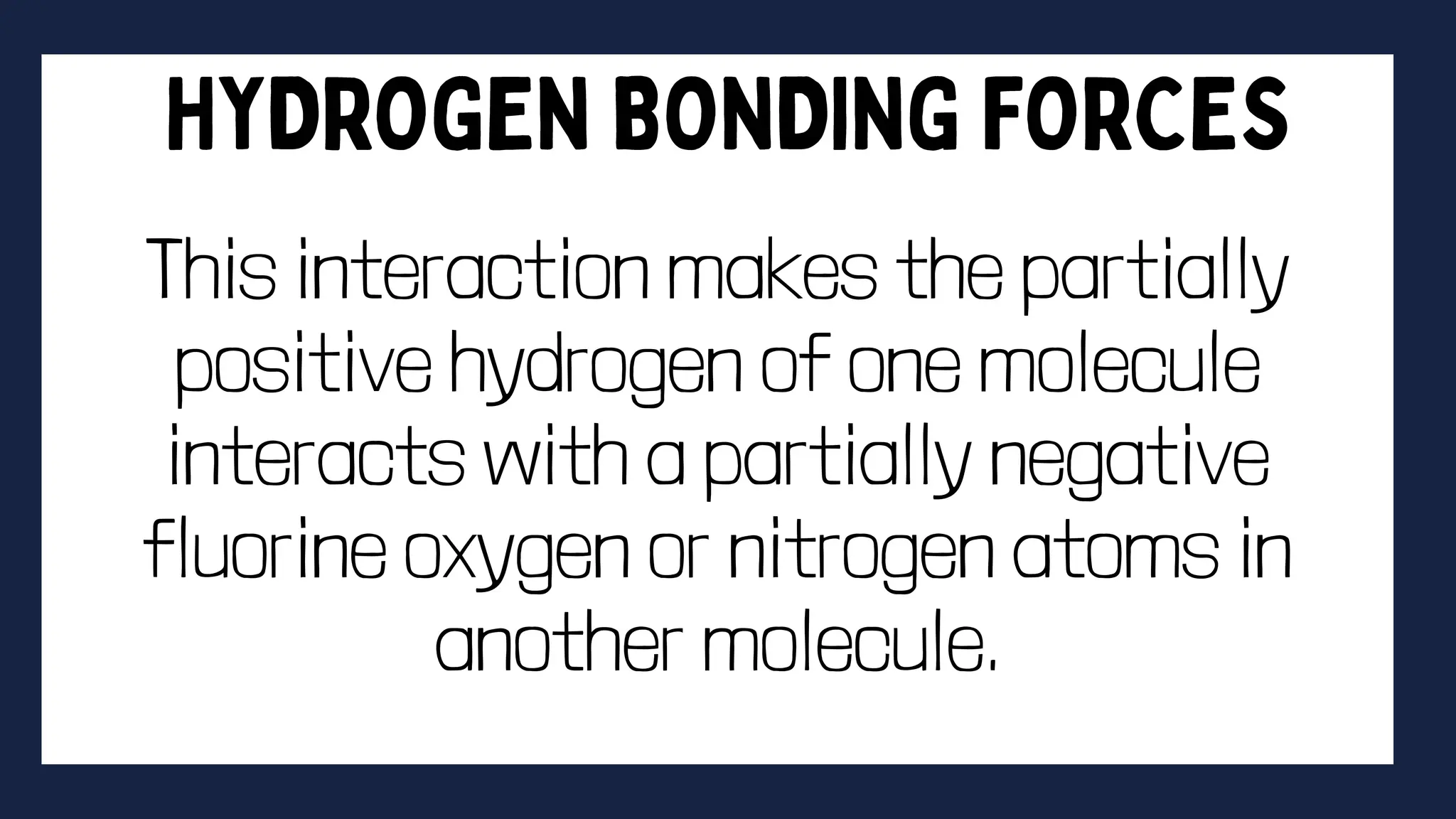
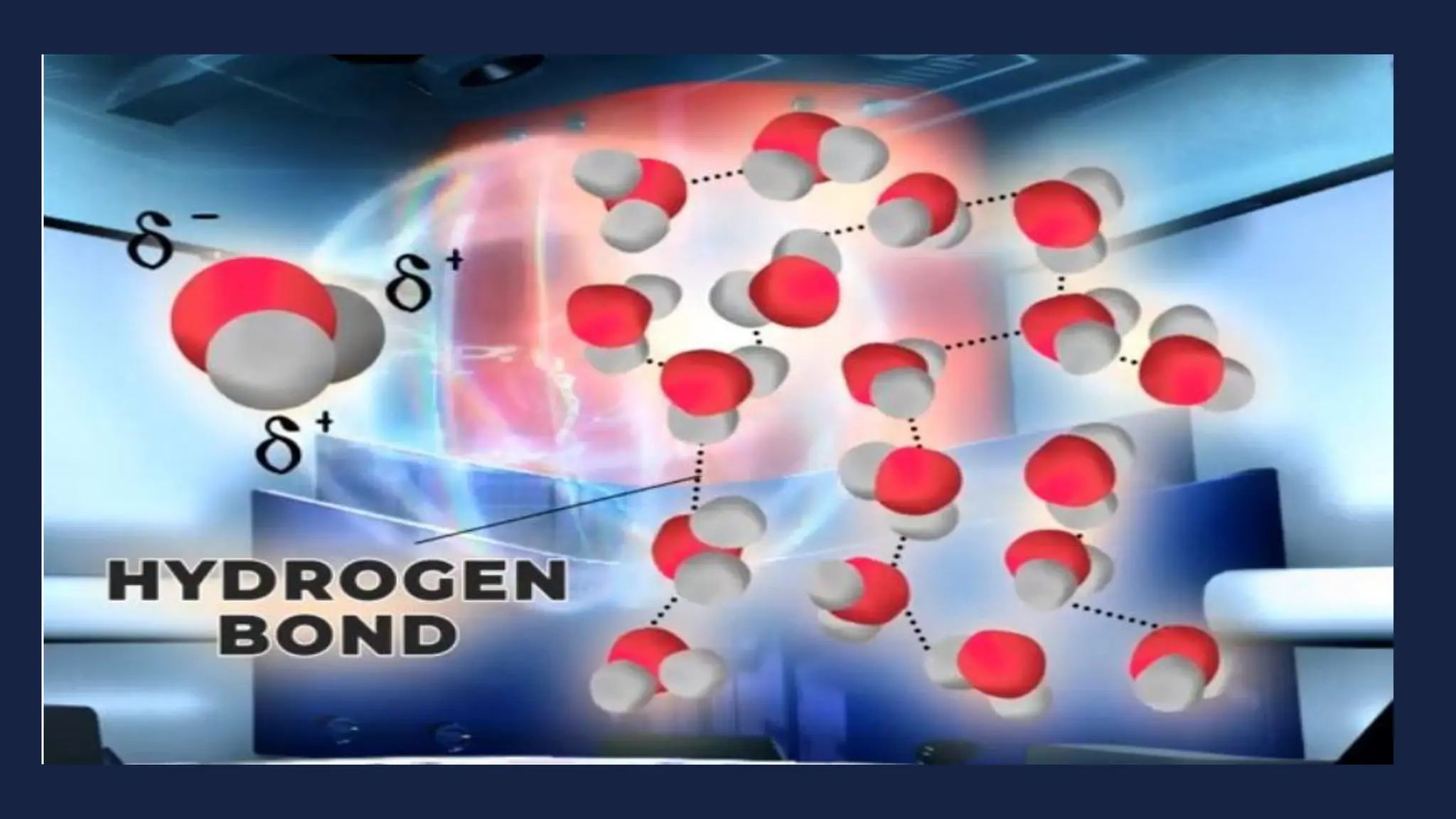
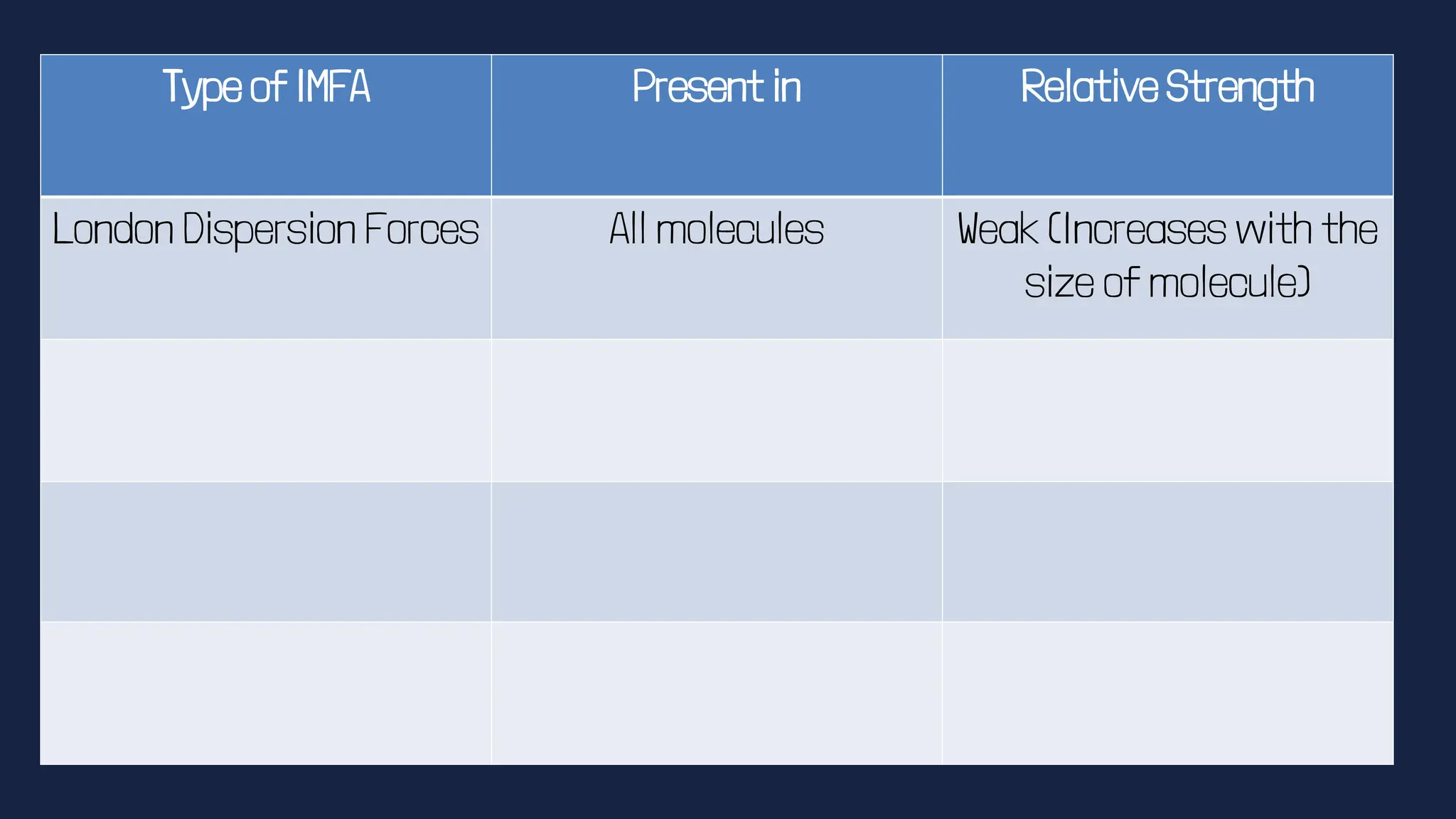
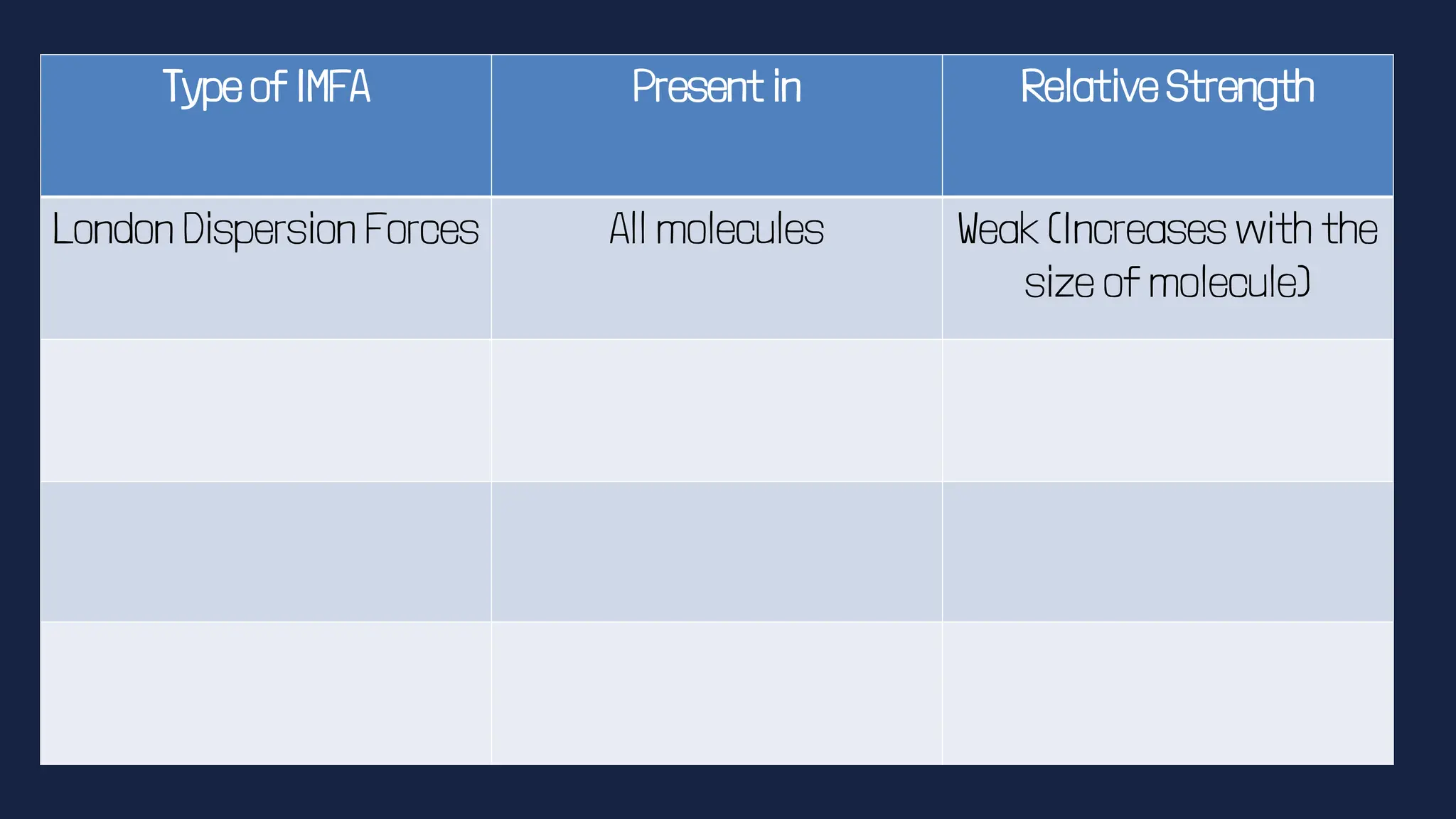
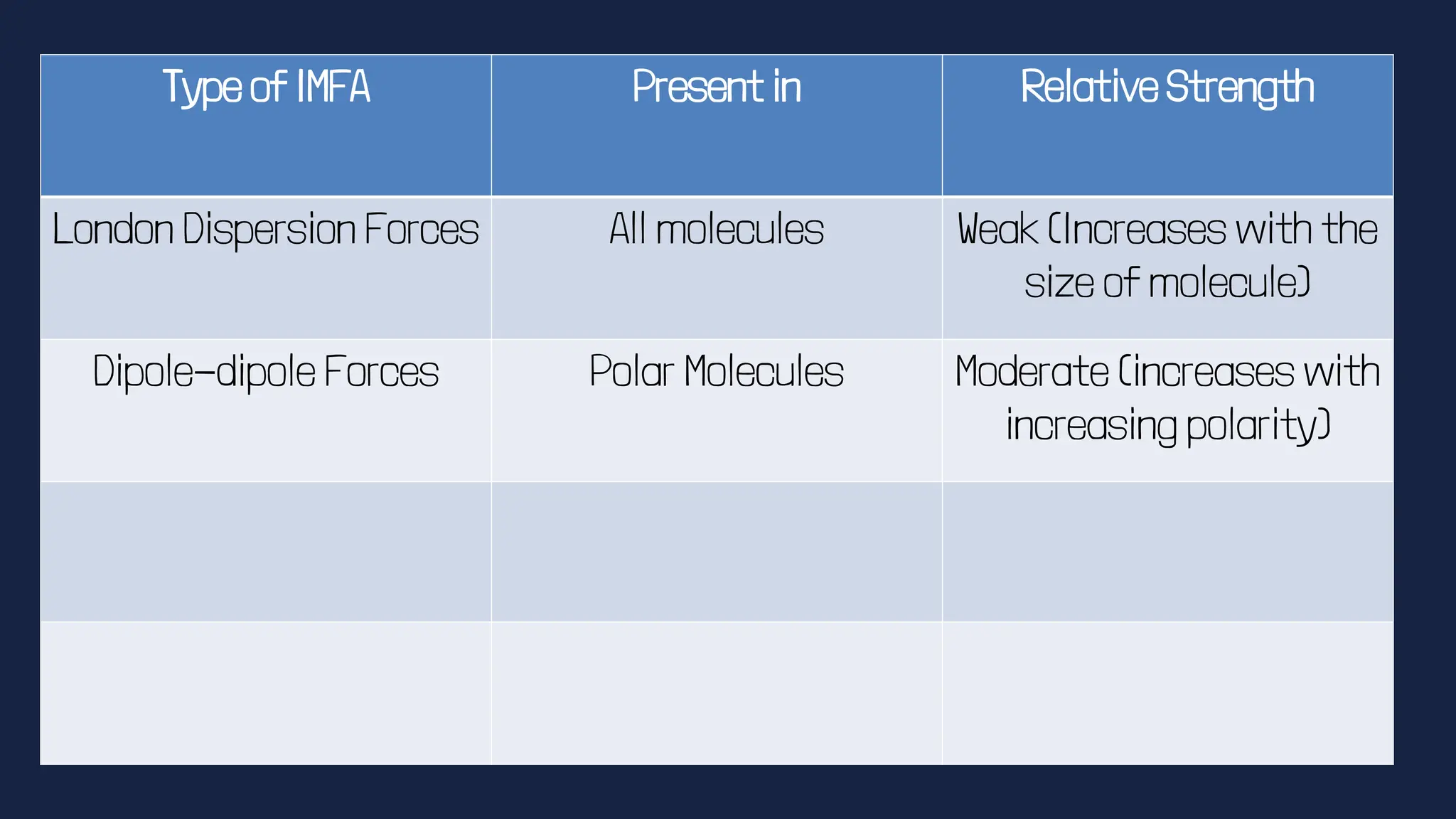
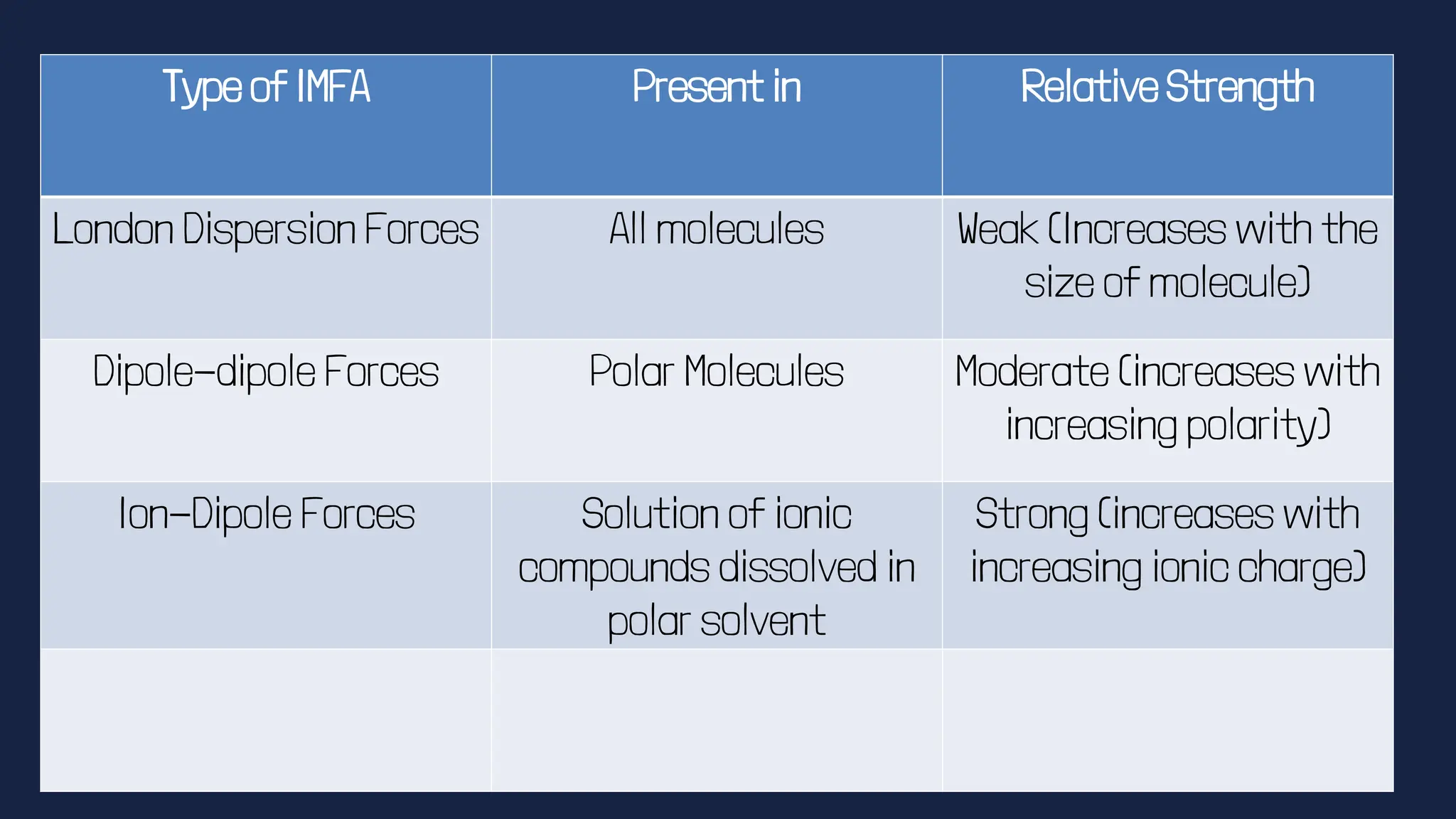
This document discusses intermolecular forces. It begins by defining adhesion and cohesion. It then discusses the four main types of intermolecular forces - London dispersion forces, dipole-dipole forces, ion-dipole forces, and hydrogen bonding. London dispersion forces are the weakest and exist between all molecules. Dipole-dipole forces are moderate in strength and exist between polar molecules. Ion-dipole forces are strong and result from attraction between ions and polar molecules. Hydrogen bonding is the strongest type and occurs when hydrogen is bonded to fluorine, oxygen, or nitrogen. The strength of intermolecular forces influences various physical properties like boiling point and viscosity.