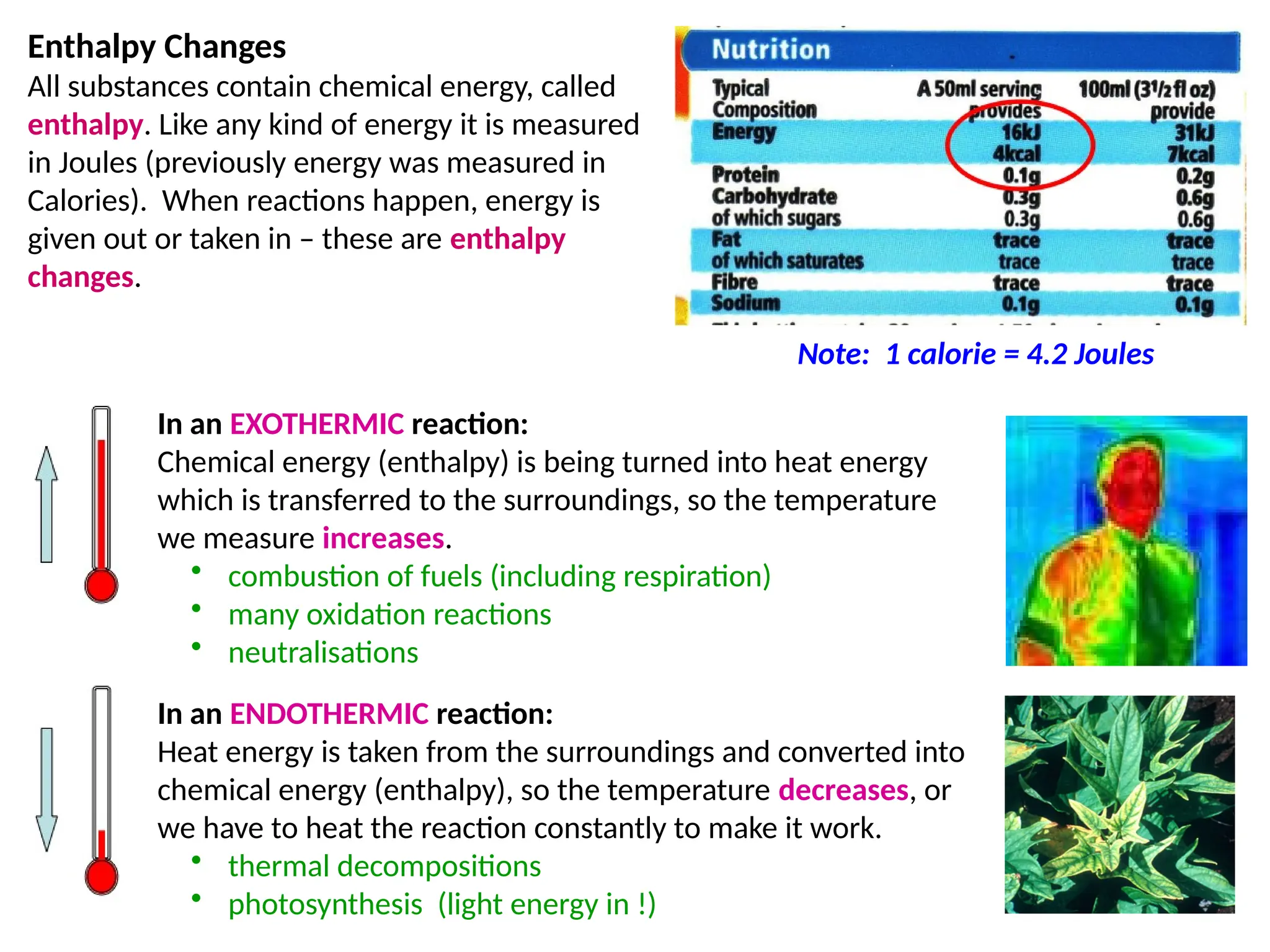
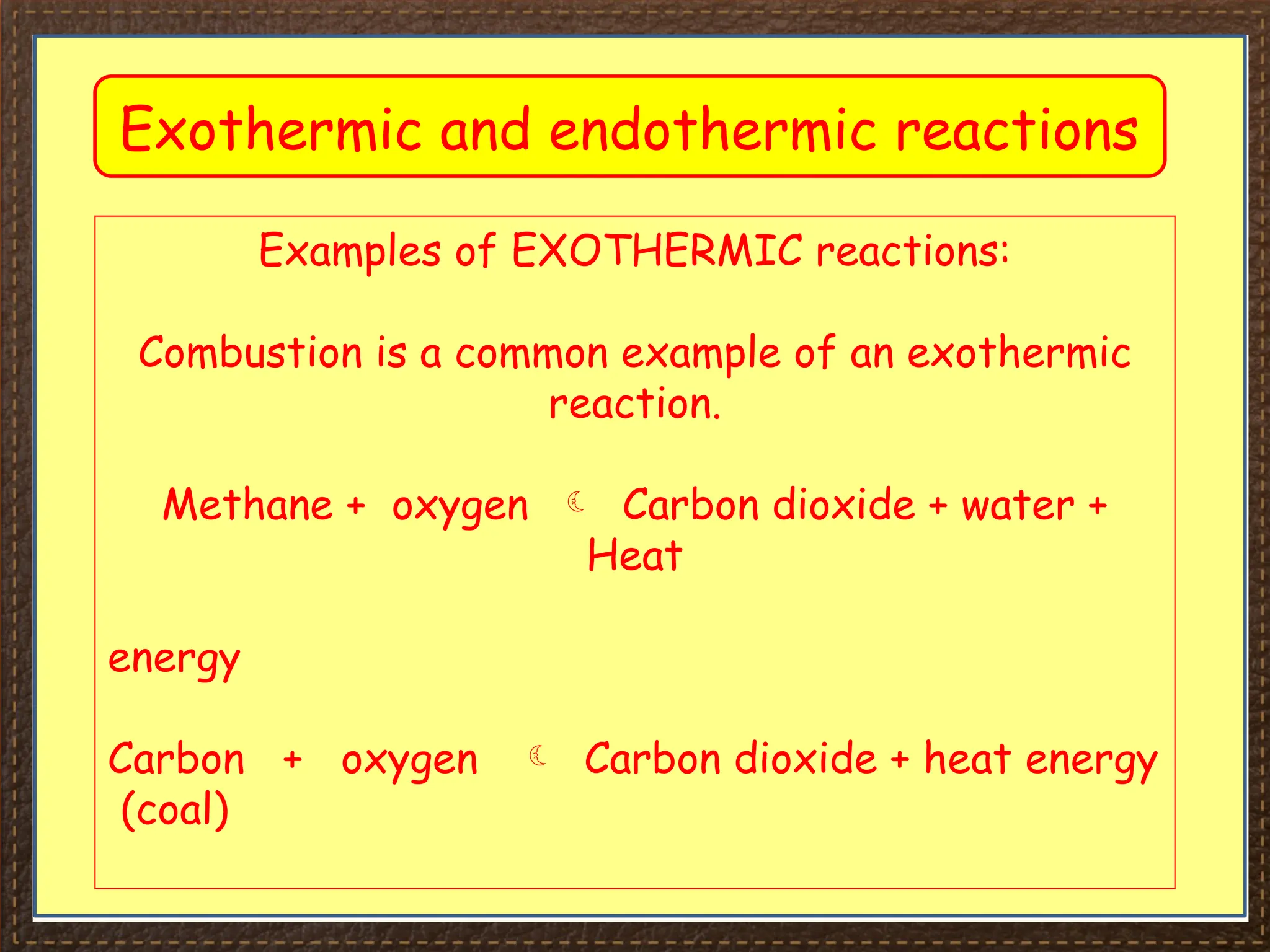
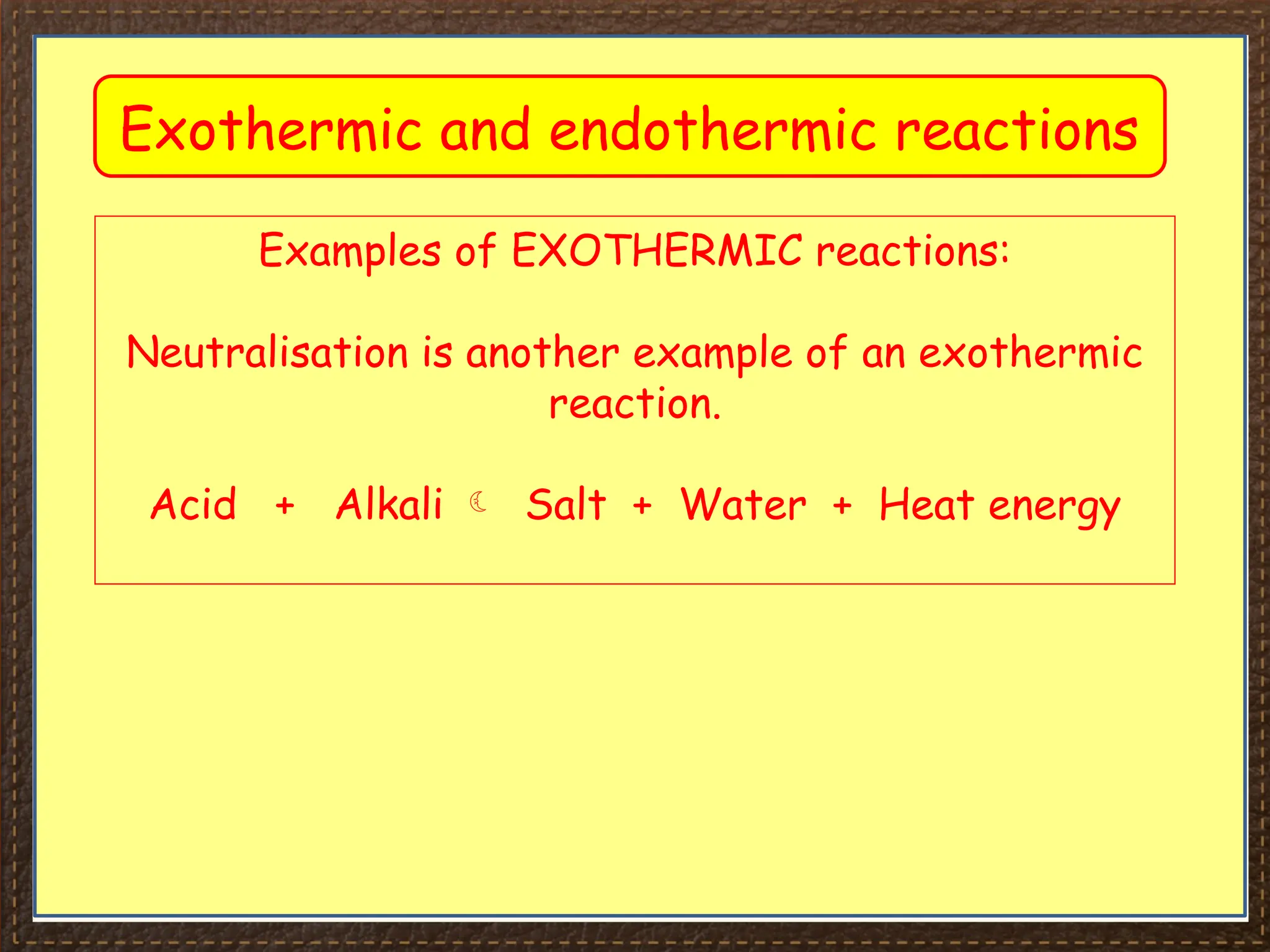
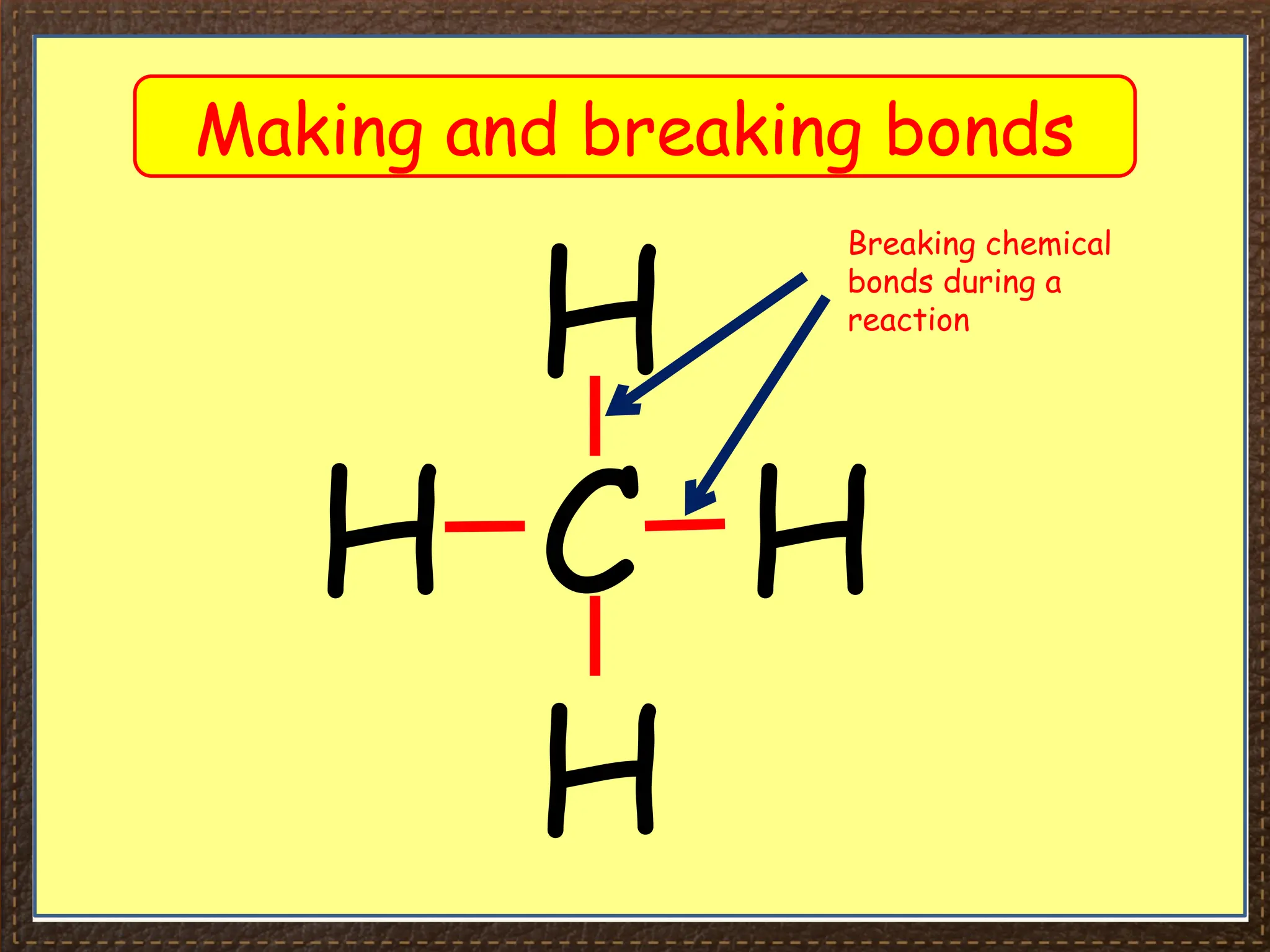
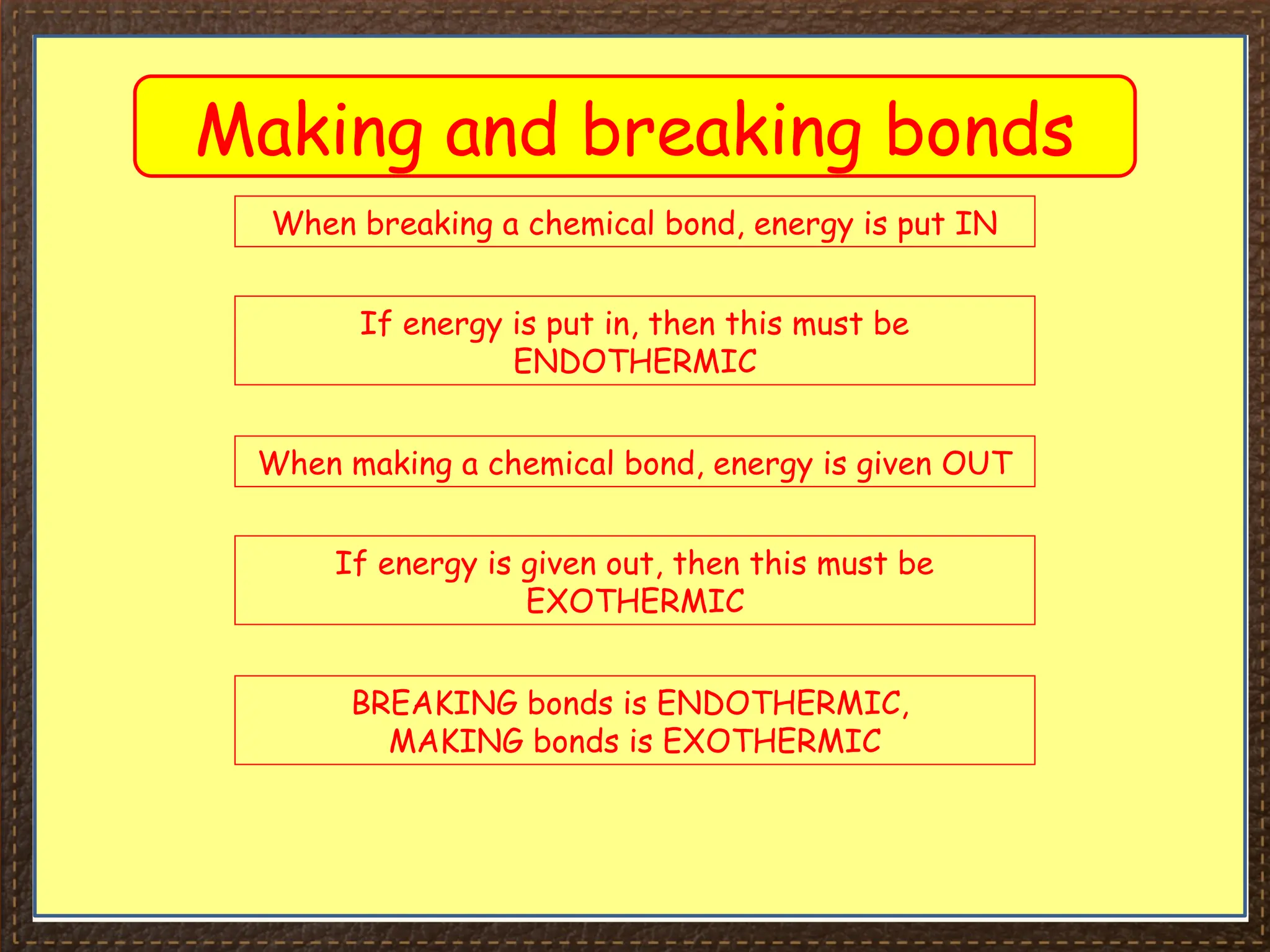
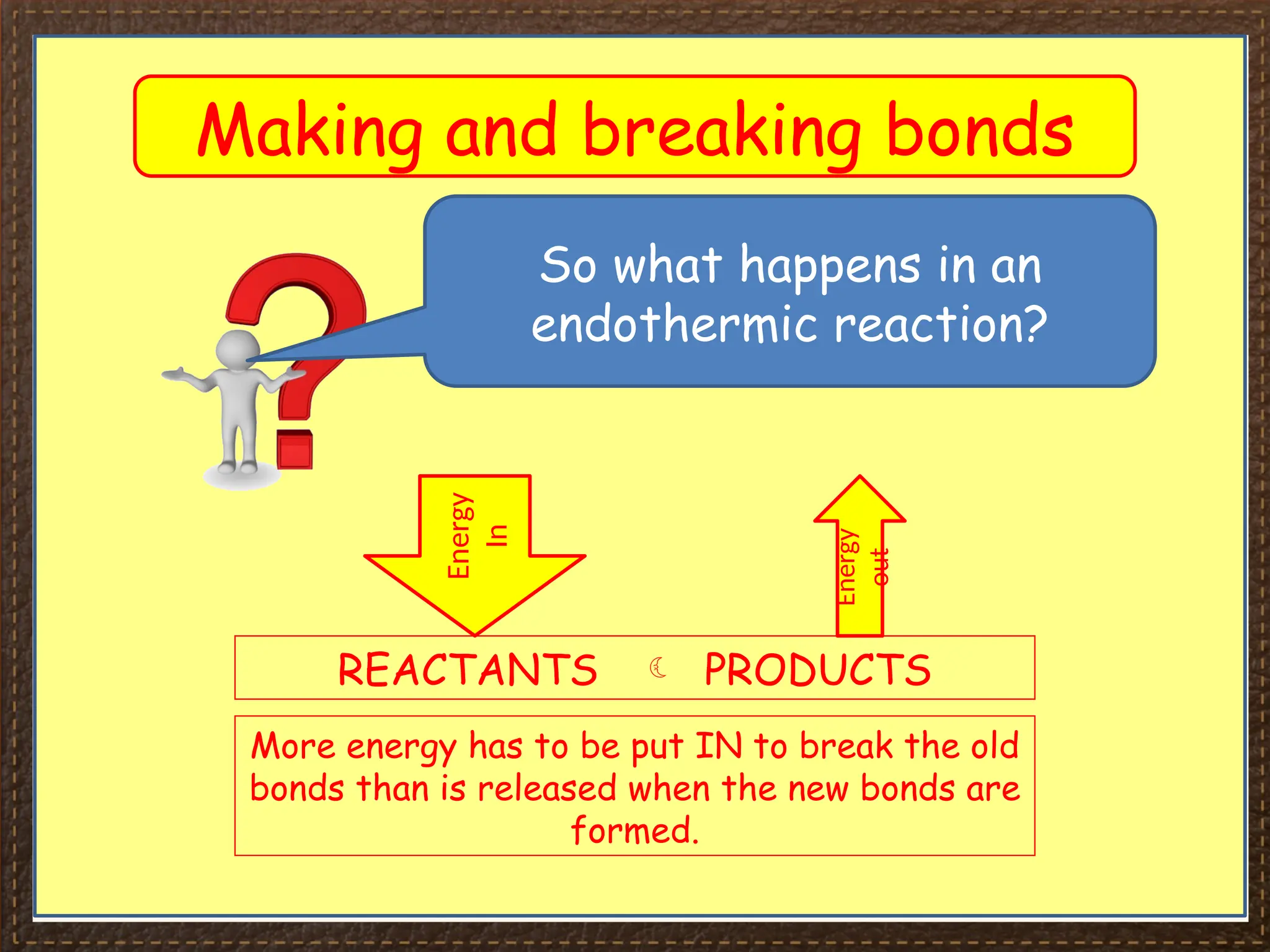
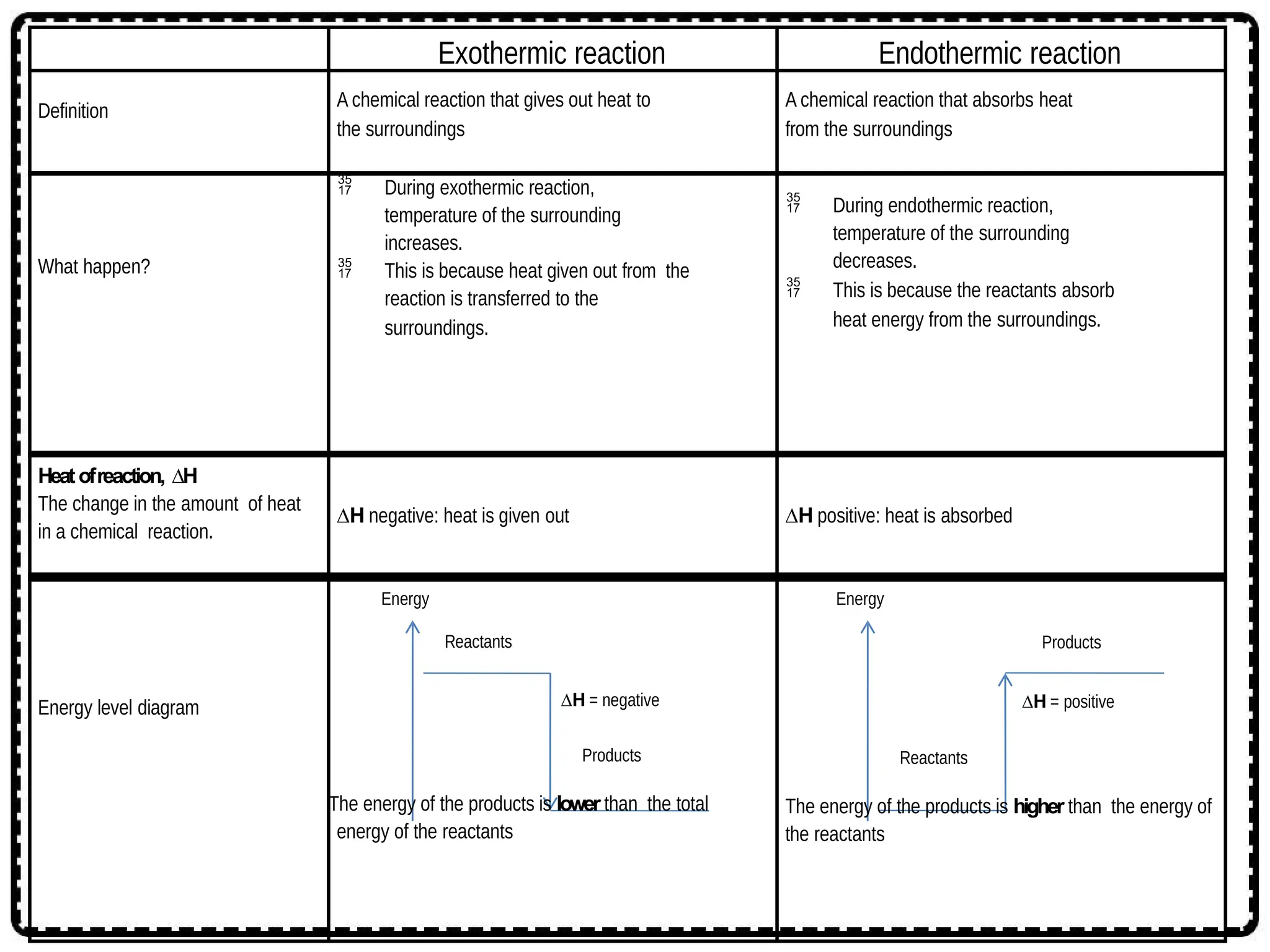
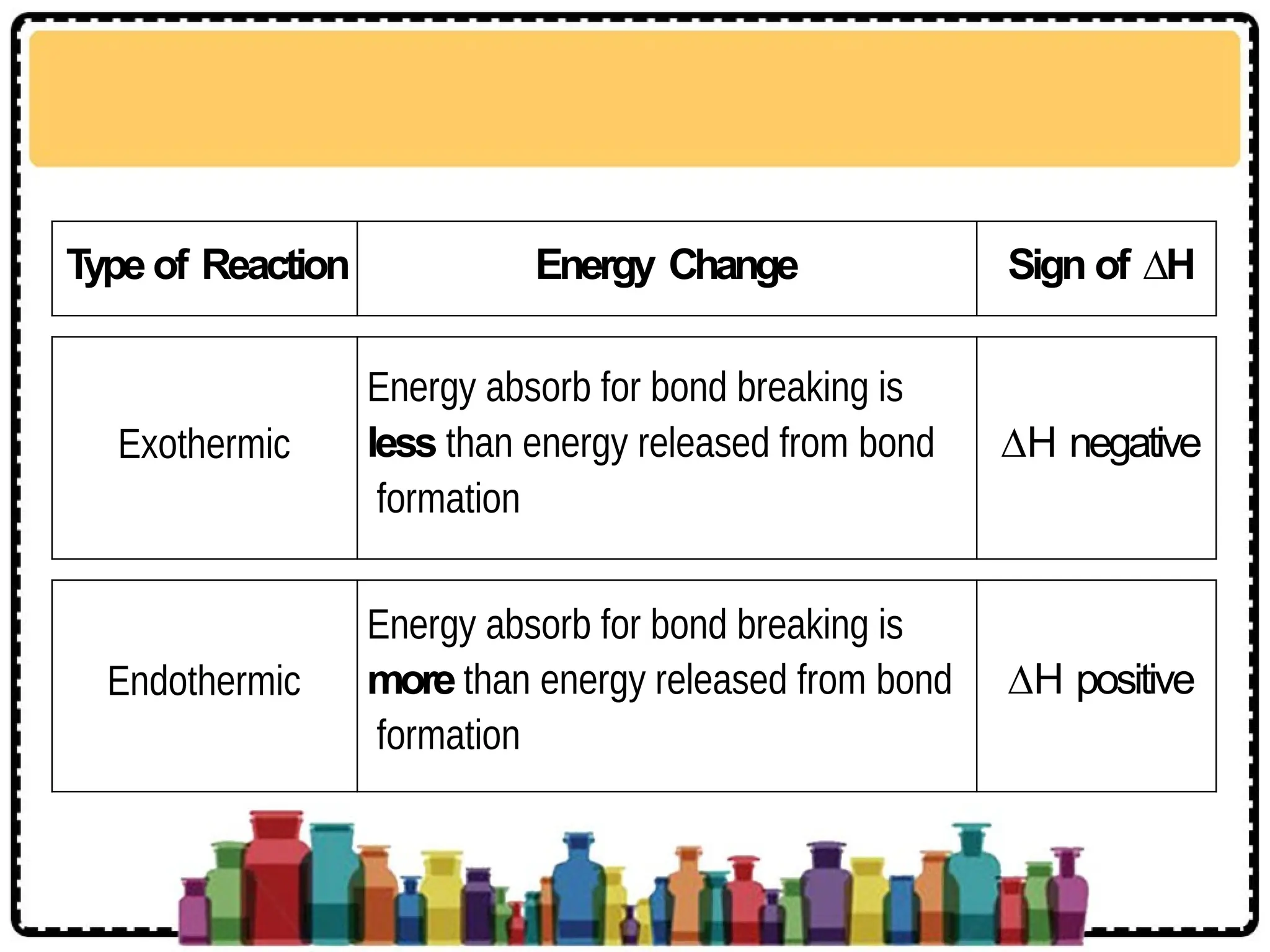
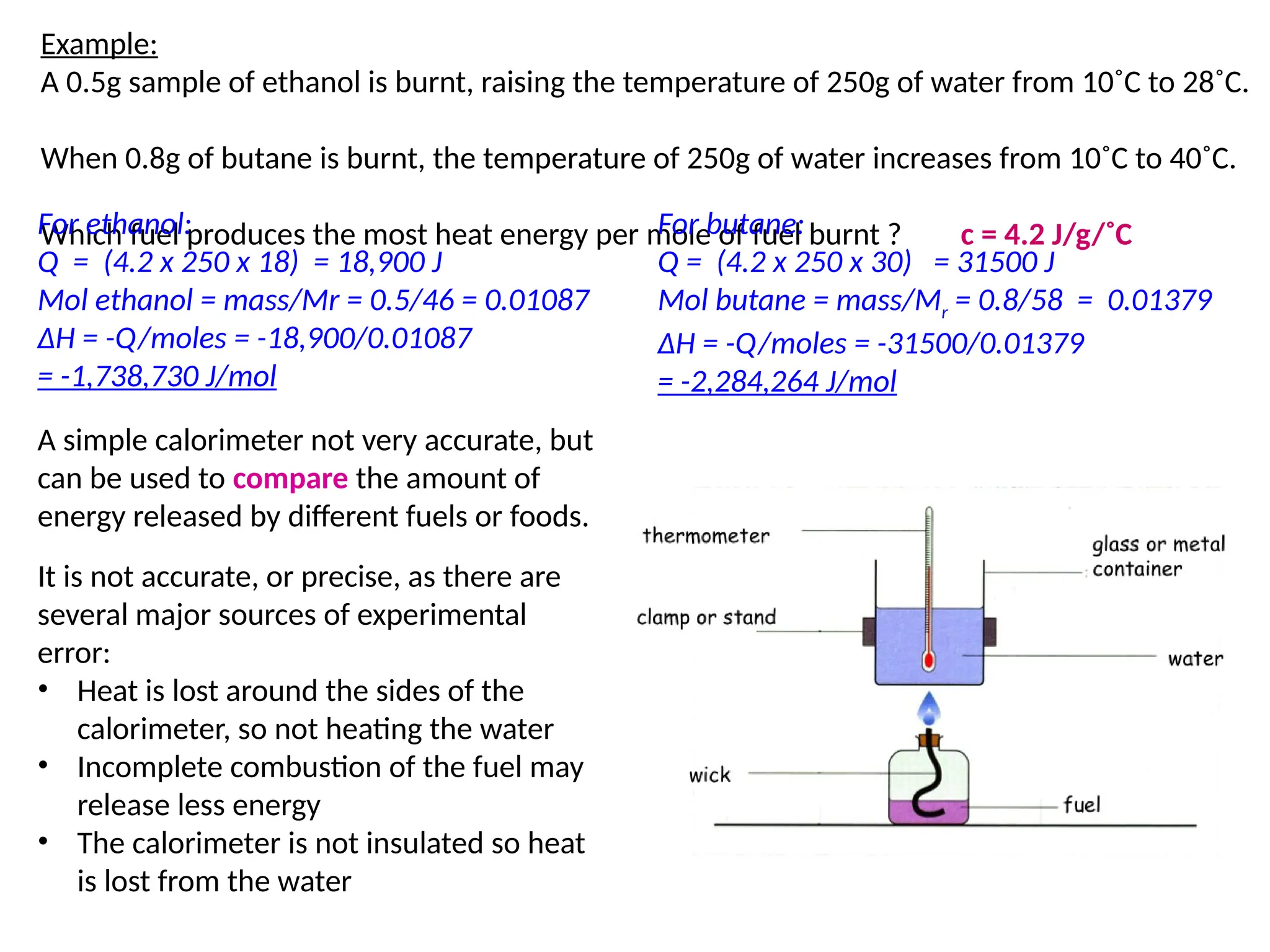
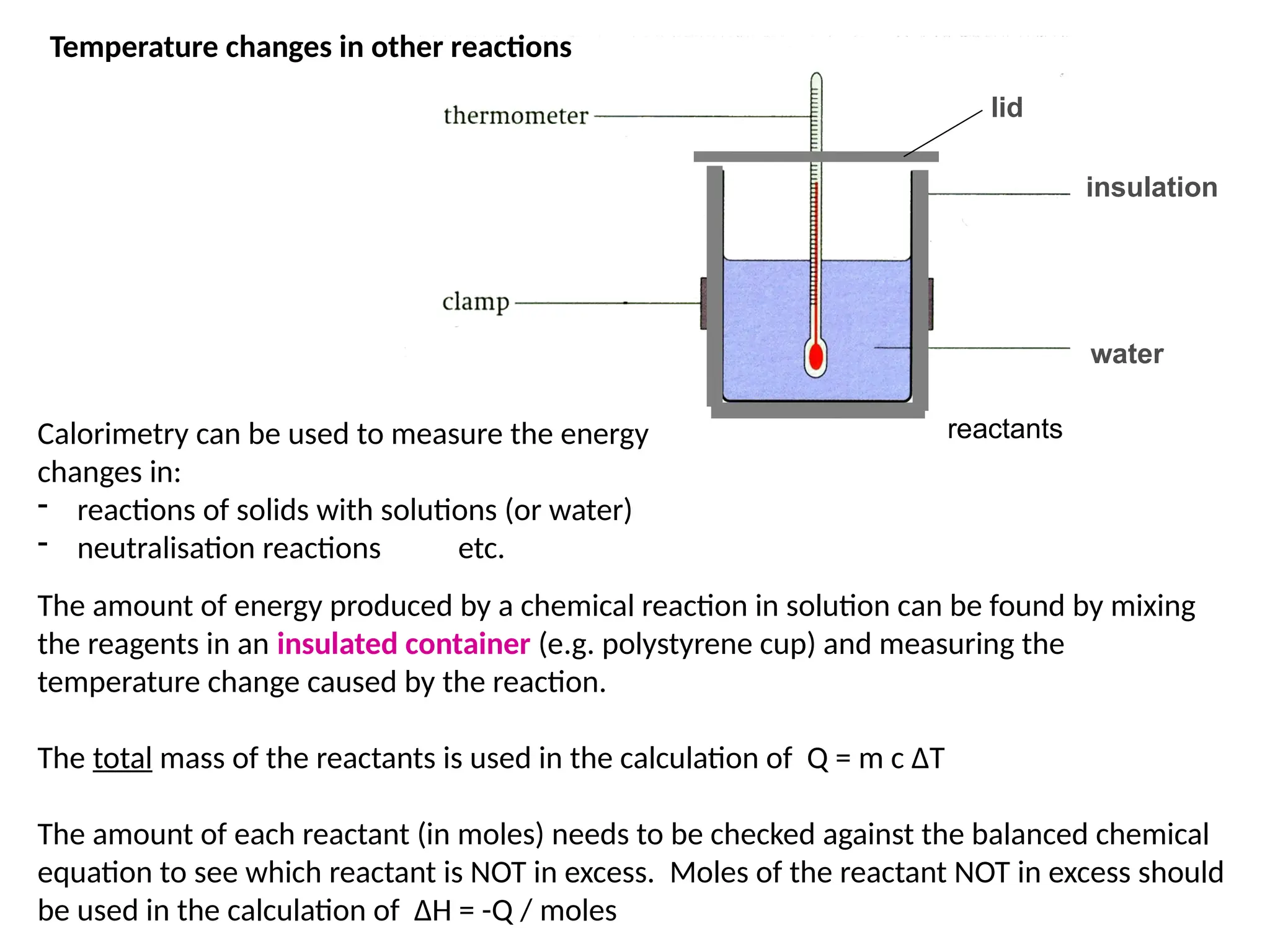
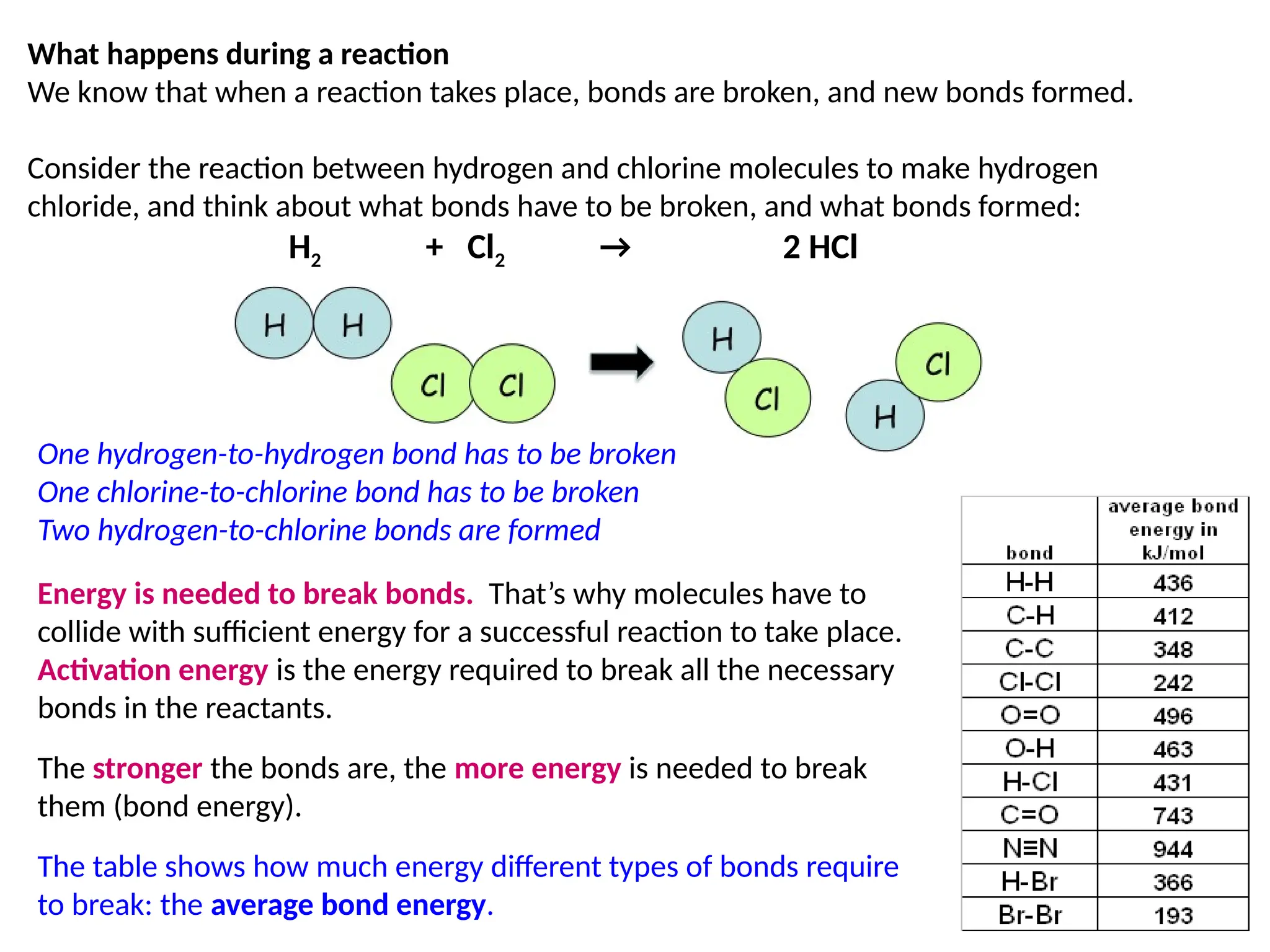
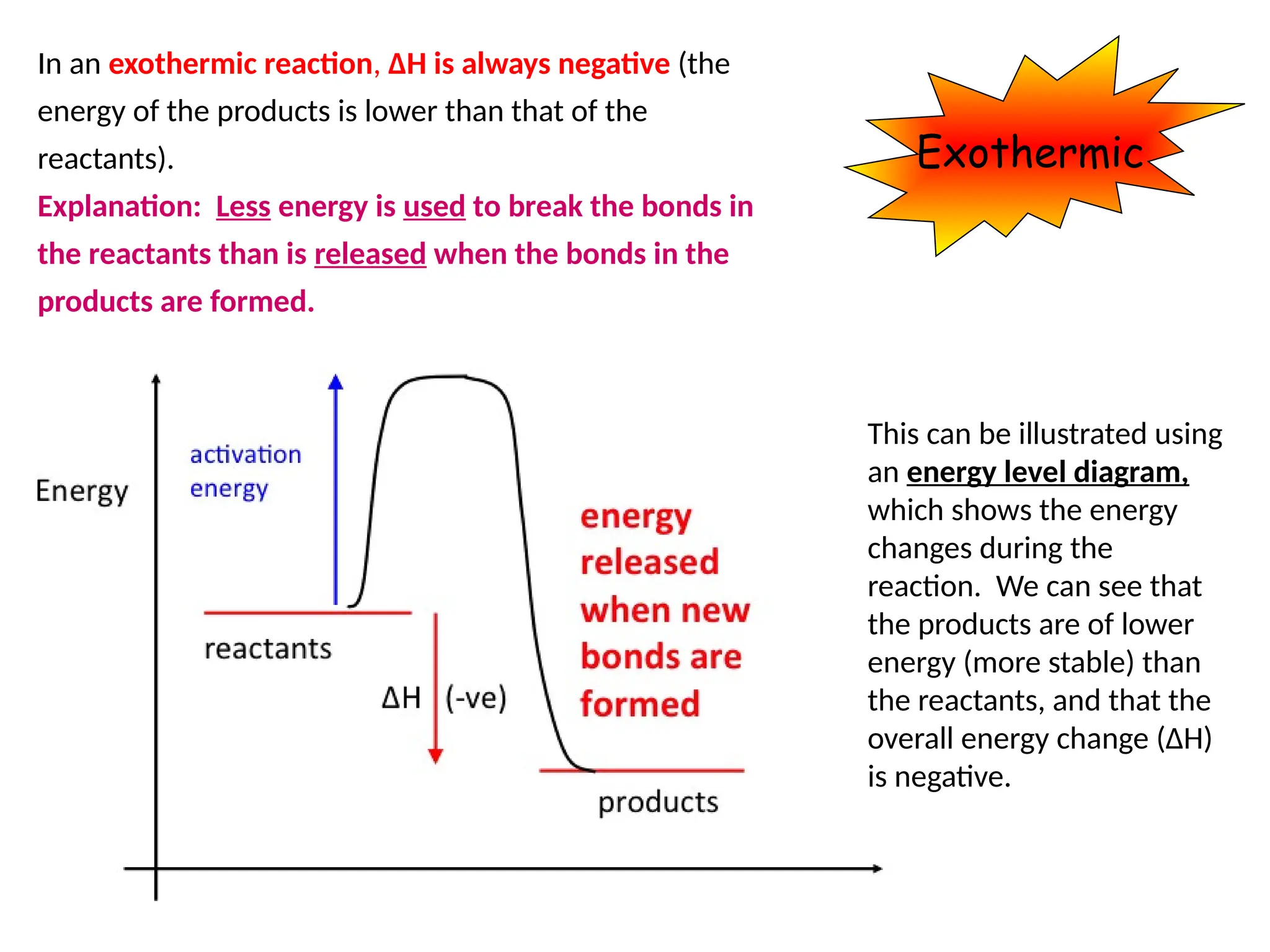
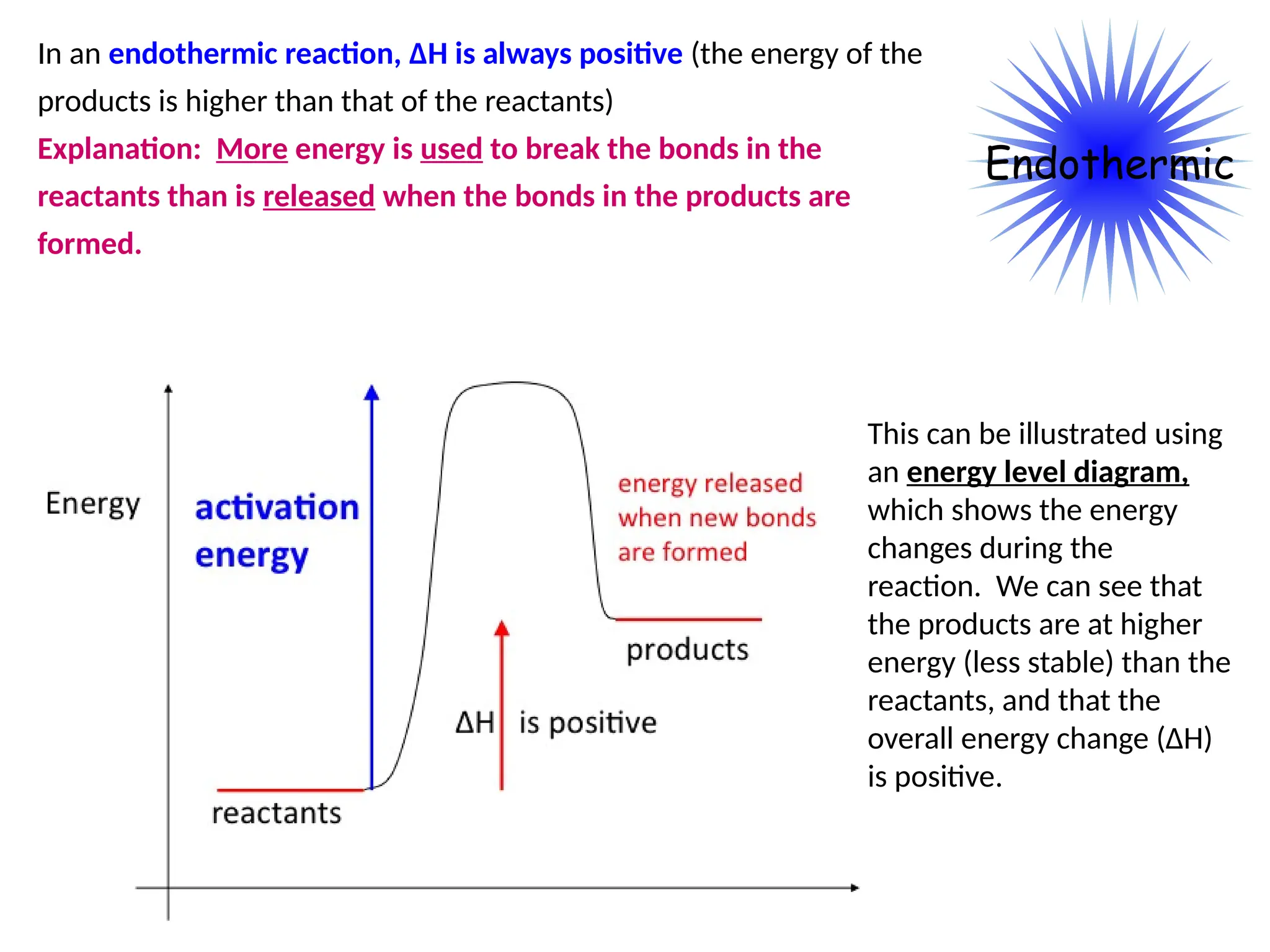
The document discusses thermochemistry, focusing on energy changes in chemical reactions, specifically exothermic and endothermic reactions. It describes how exothermic reactions release heat and increase surrounding temperature, while endothermic reactions absorb heat and decrease temperature, including practical uses for both types of reactions. Additionally, it covers energy calculations, calorimetry, bond energy, and the significance of enthalpy changes in reactions.