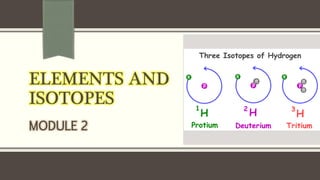
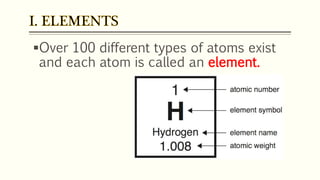
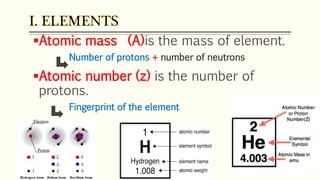
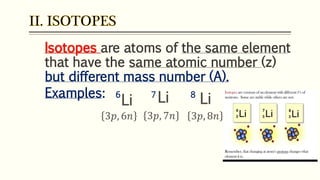
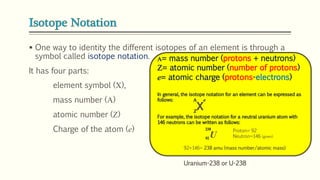
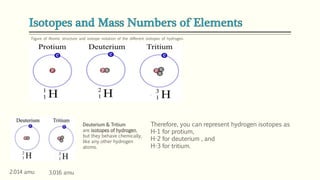
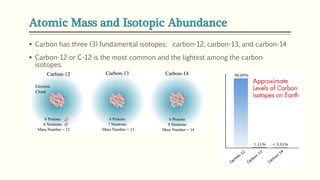
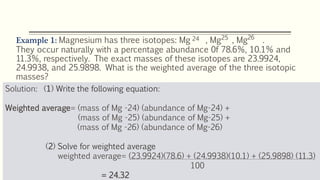
The document discusses three reactions - nucleosynthesis, fusion, and neutron capture reaction - that led to the formation of elements heavier than iron. It explains that these reactions require a certain amount of energy to proceed. It also provides an overview of elements and isotopes, defining terms like atomic number, mass number, and isotope notation. Elements heavier than iron are formed through nucleosynthesis or neutron capture reactions, while isotopes are formed through these reactions or radioactive decay and can be represented using isotope notation.