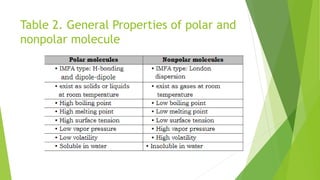
This document discusses the polarity of molecules and how it relates to properties like solubility and miscibility. It defines polarity as a separation of electric charge within a molecule, leading to partial charges. Polar molecules contain polar bonds with an electrostatic difference. Solubility is defined as a substance dissolving in a solvent, while miscibility is the ability of liquids to mix in all proportions. The document states that "like dissolves like" meaning polar substances dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. It provides examples of polar and nonpolar substances and whether they would mix or form separate layers. The strength of intermolecular forces affects properties such as boiling point, melting point, vapor pressure and