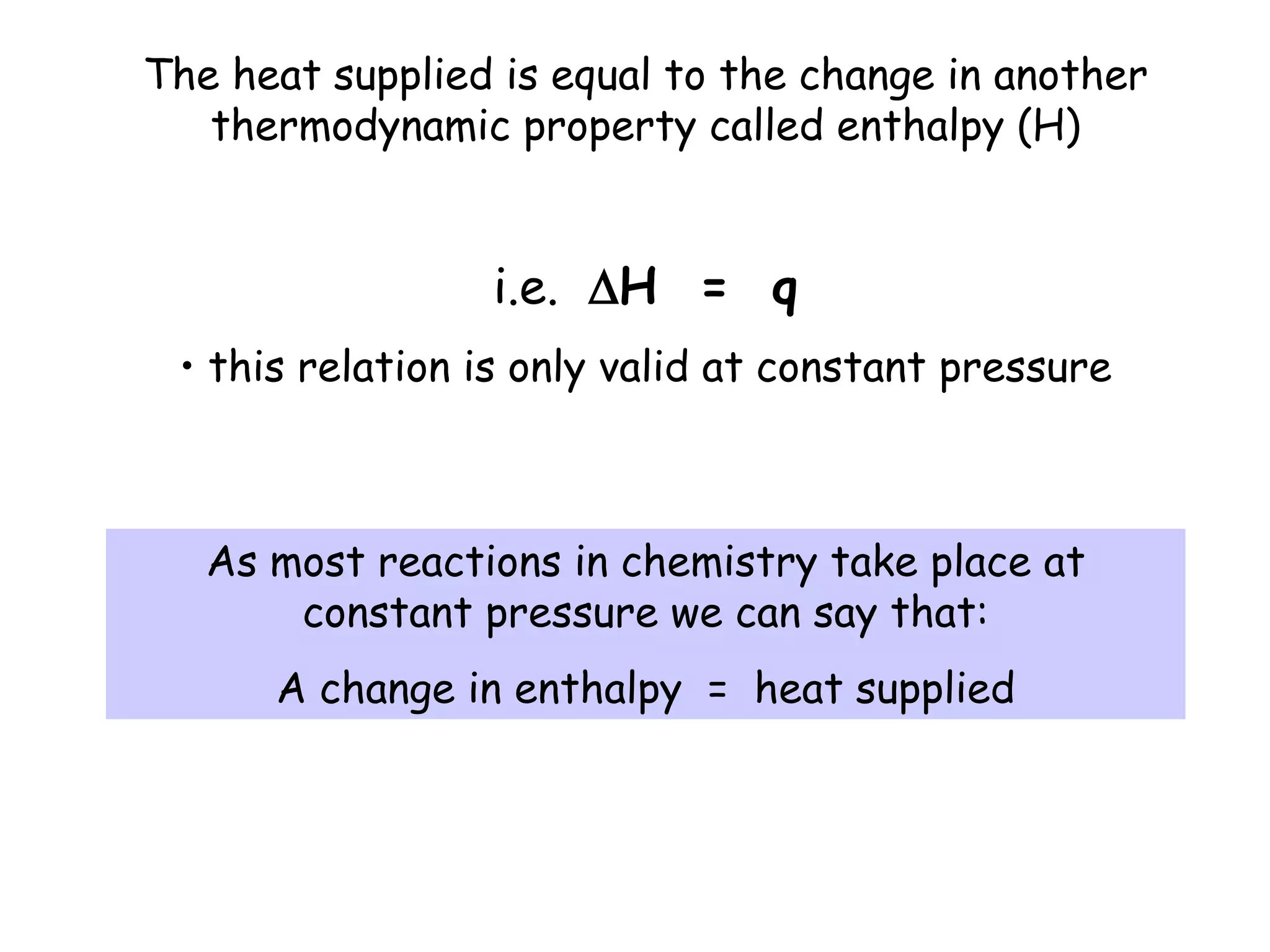
Thermochemistry is the study of heat released or absorbed by chemical reactions. Combustion reactions like burning methane are exothermic, releasing heat. The internal energy of a system can change as heat or work, and is constant for isolated systems by the first law of thermodynamics. Exothermic reactions have a negative enthalpy change, while endothermic reactions have a positive enthalpy change. Spontaneous reactions tend to occur with an overall increase in entropy by the second law of thermodynamics.