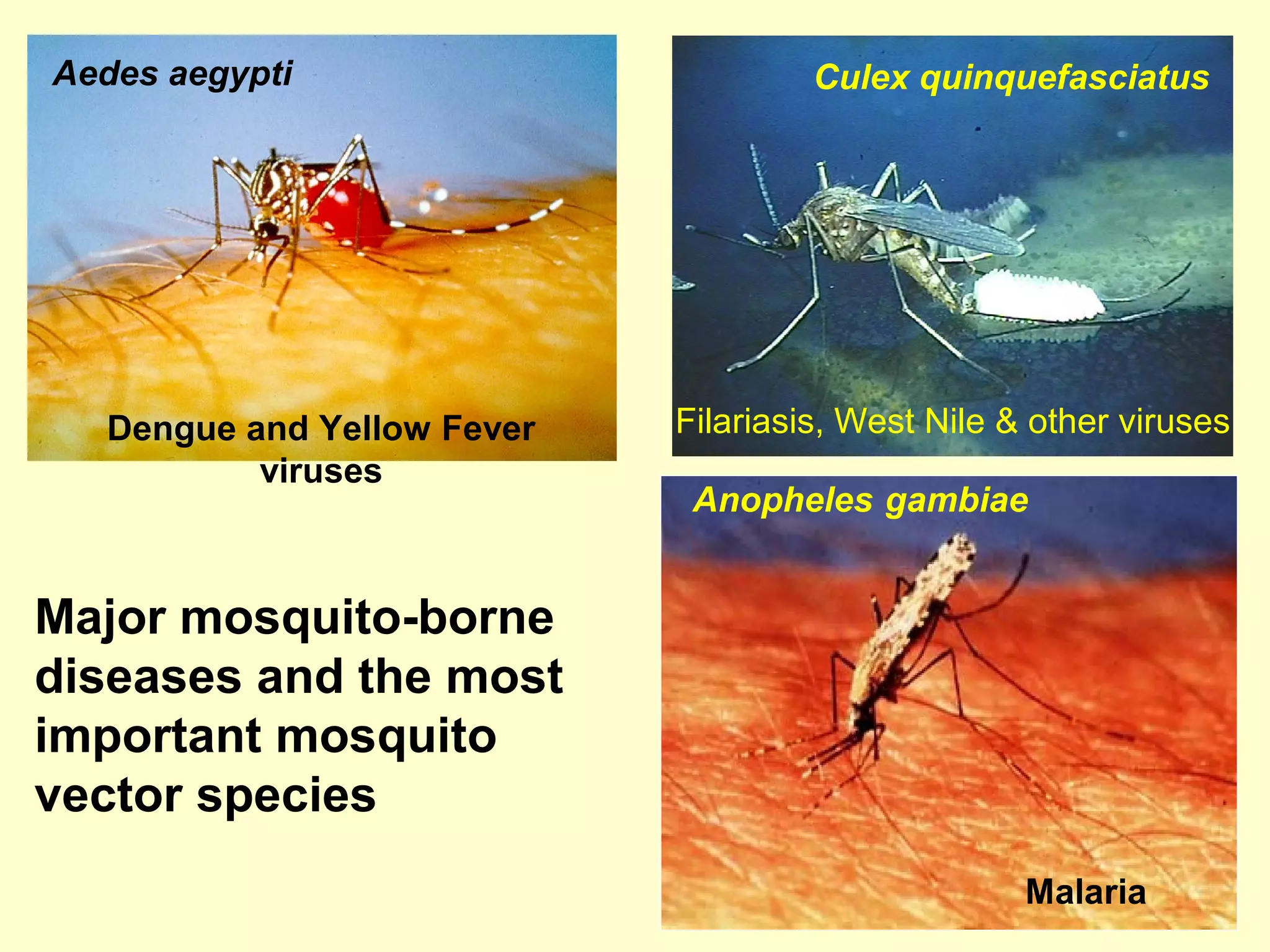
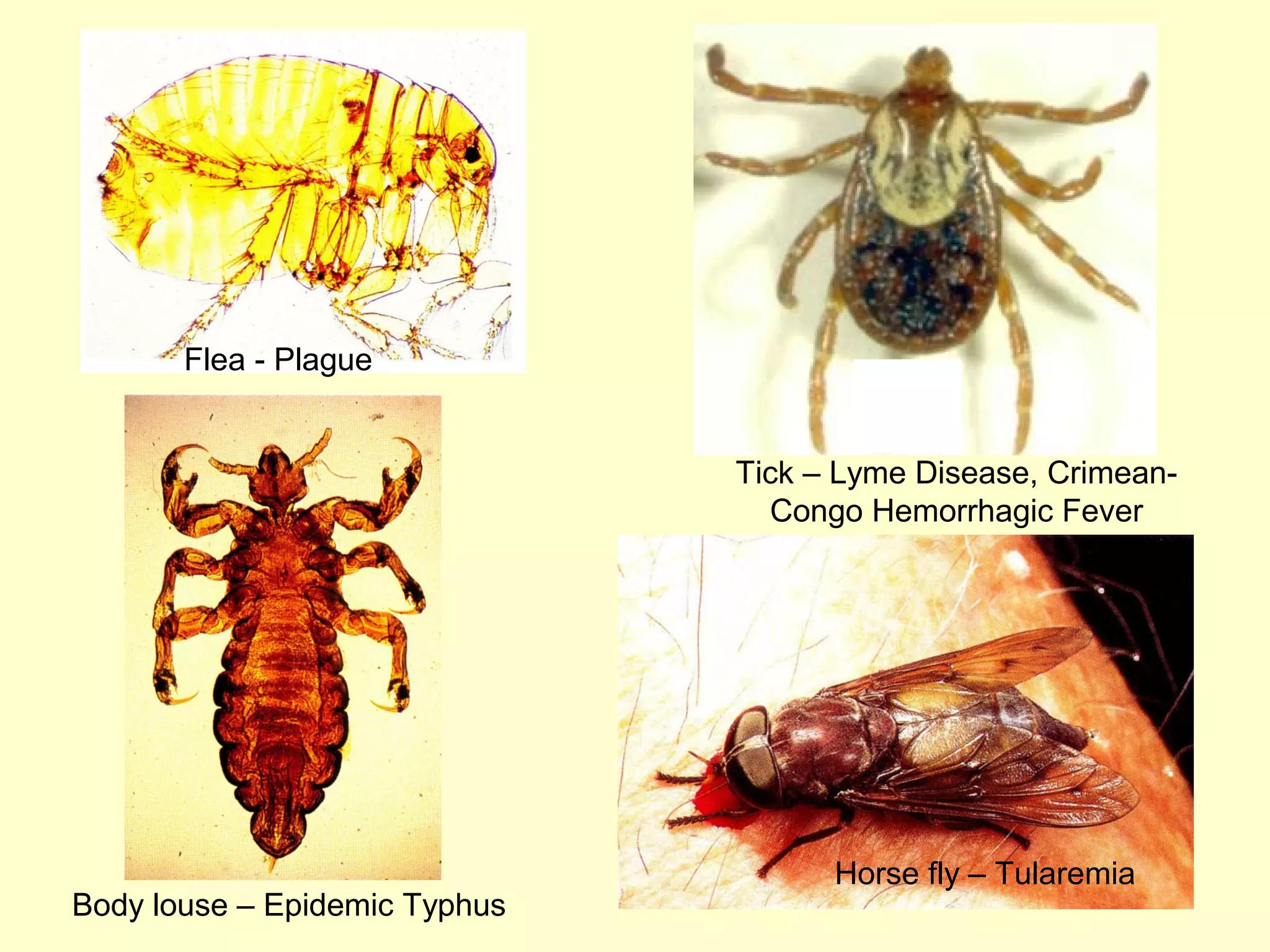
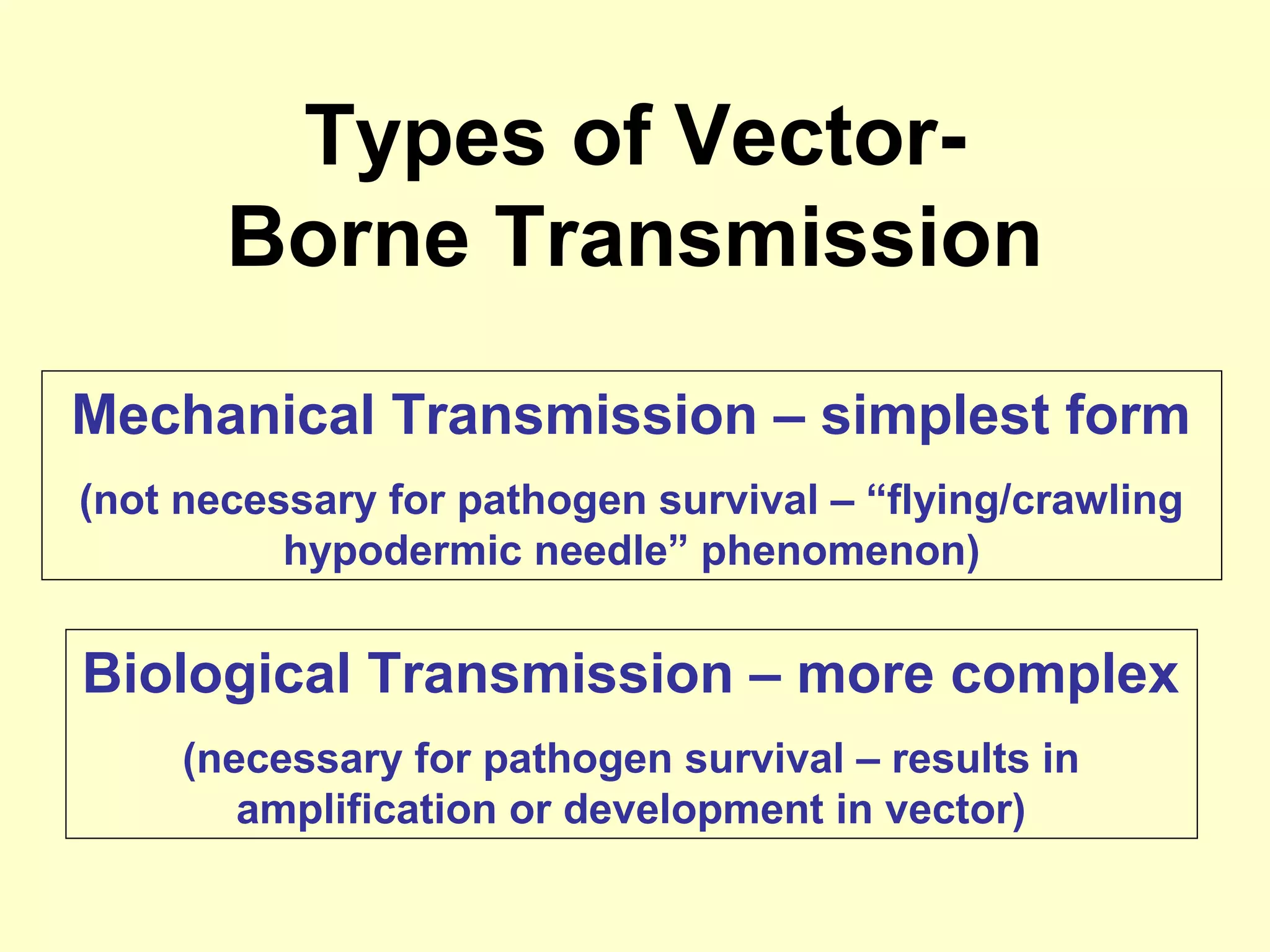
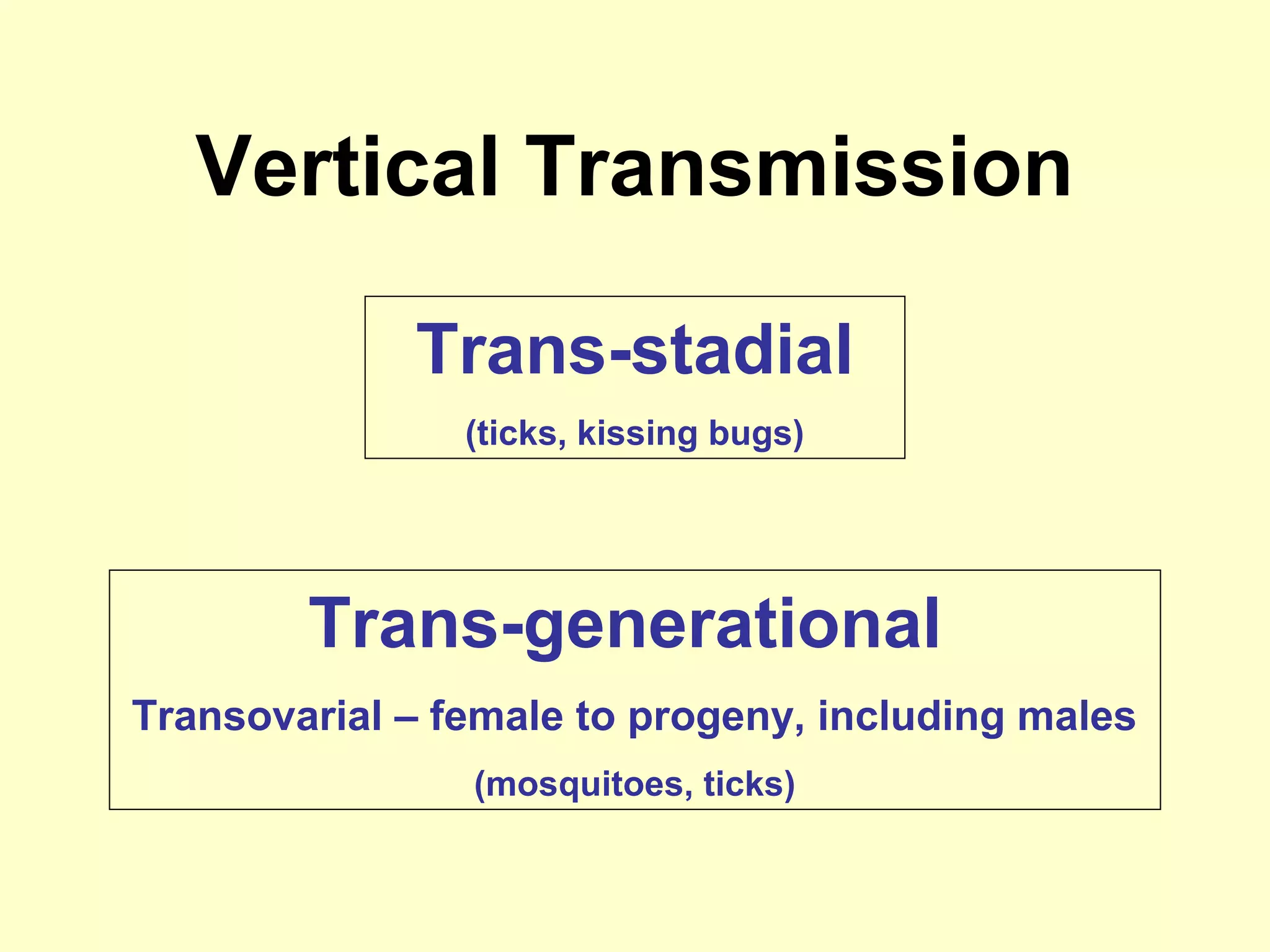
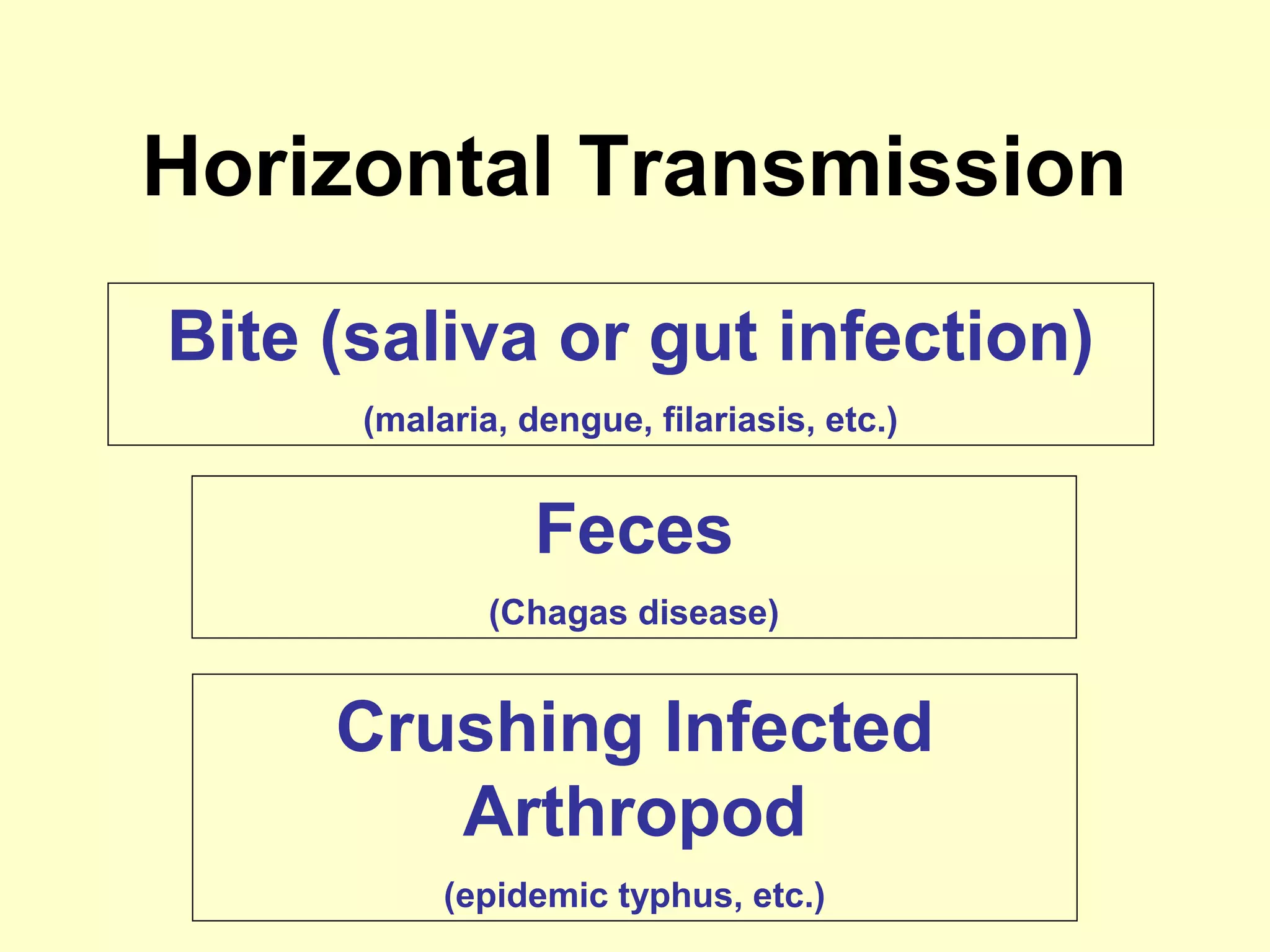
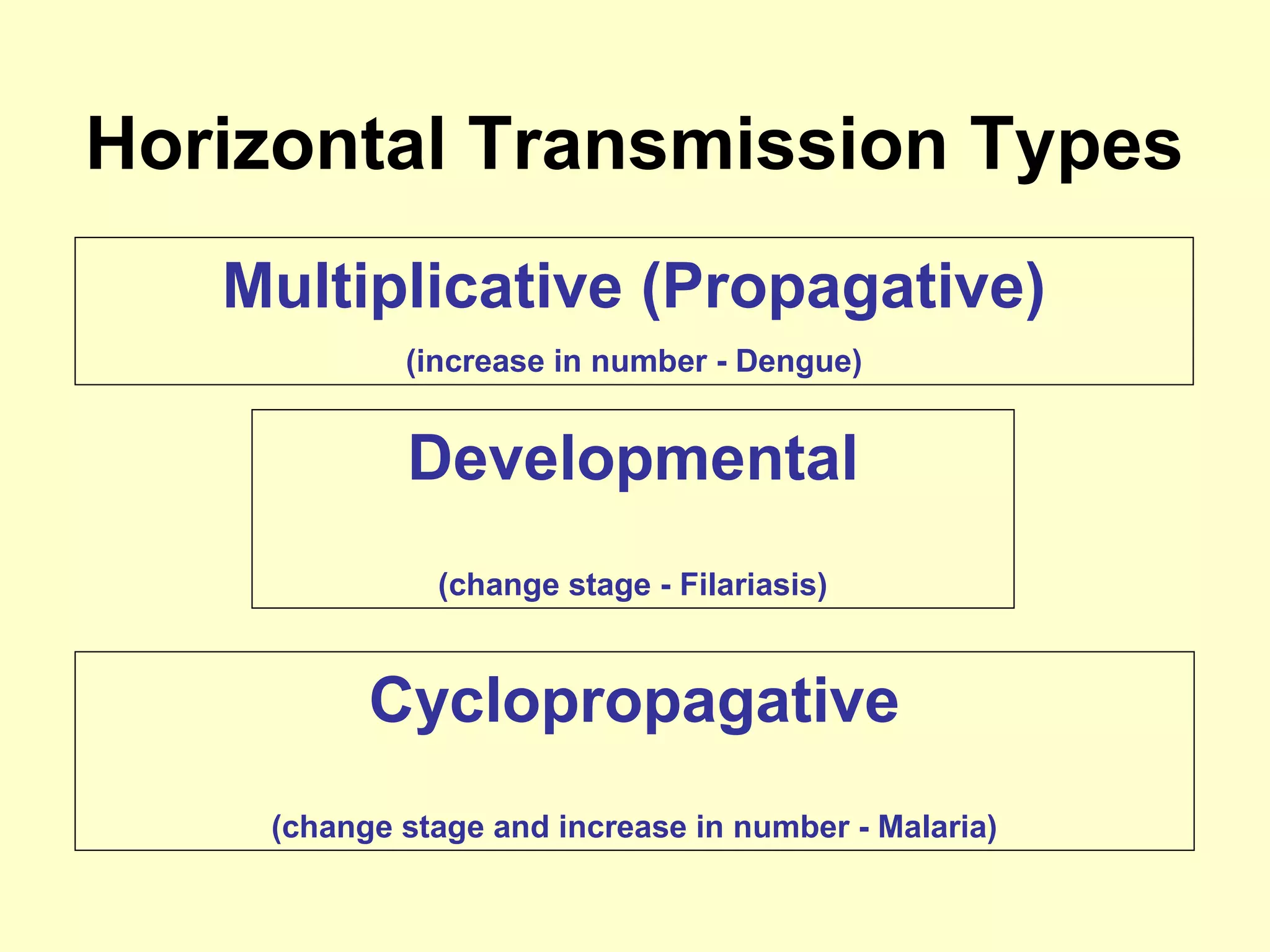
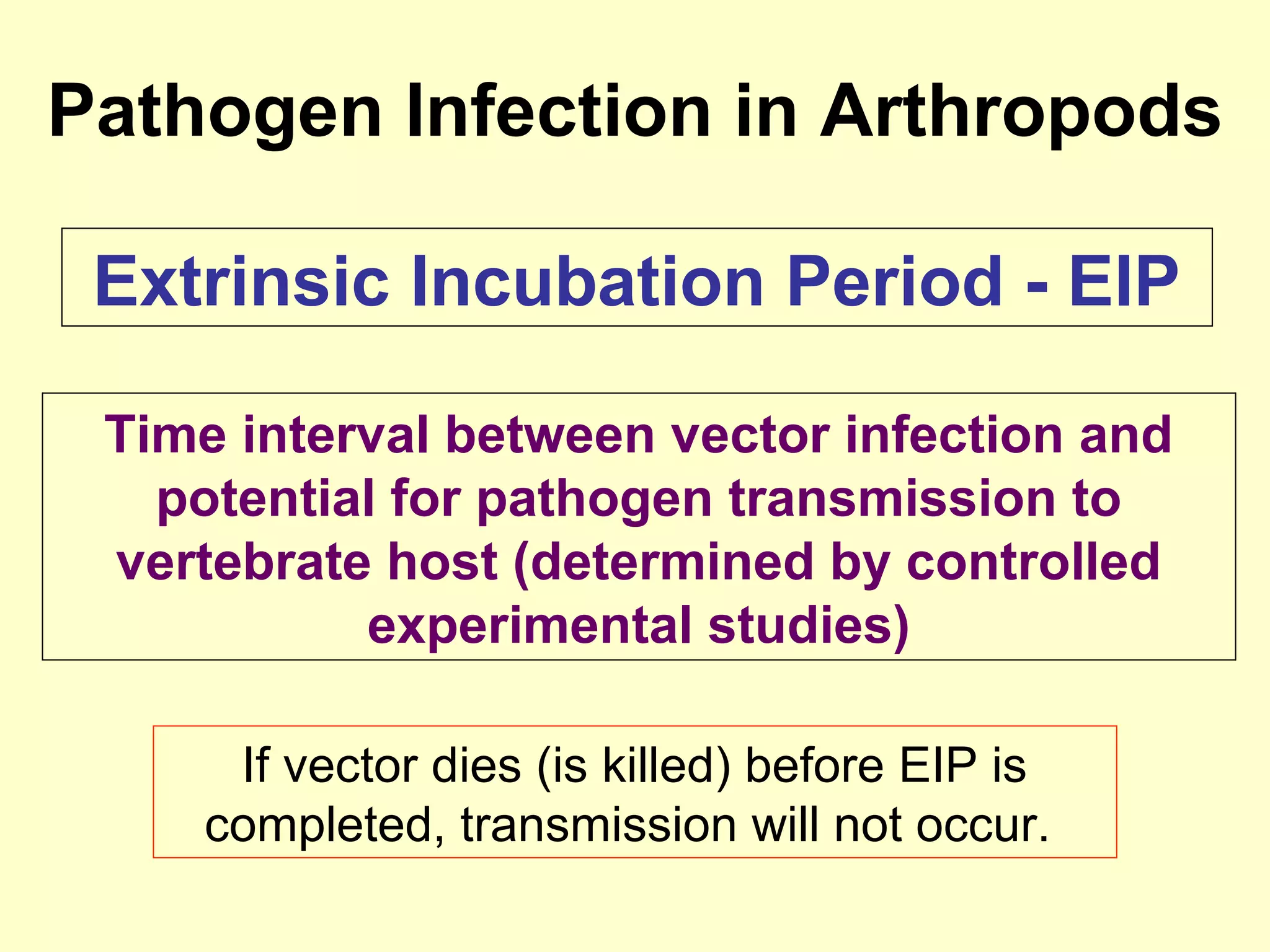
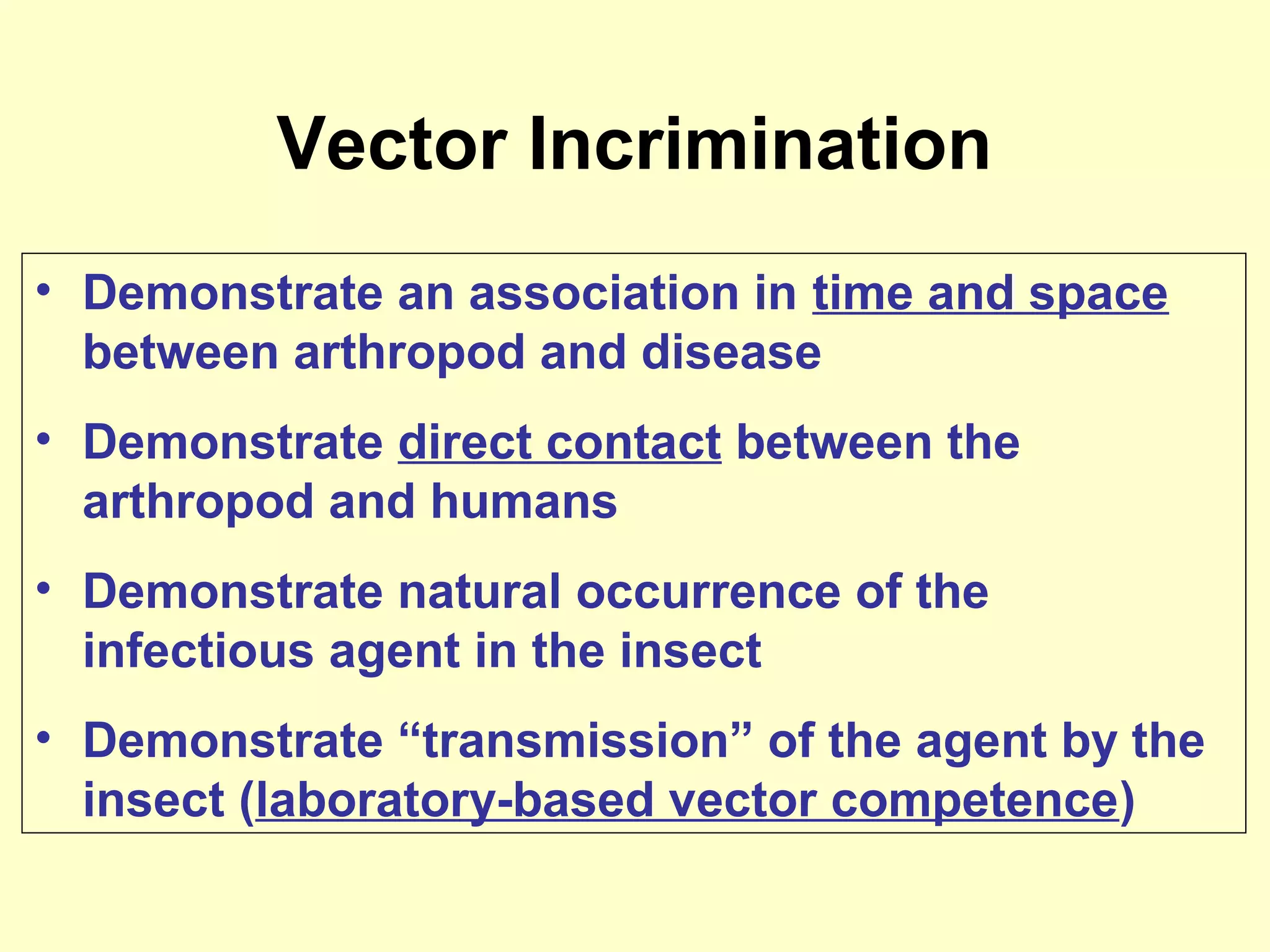
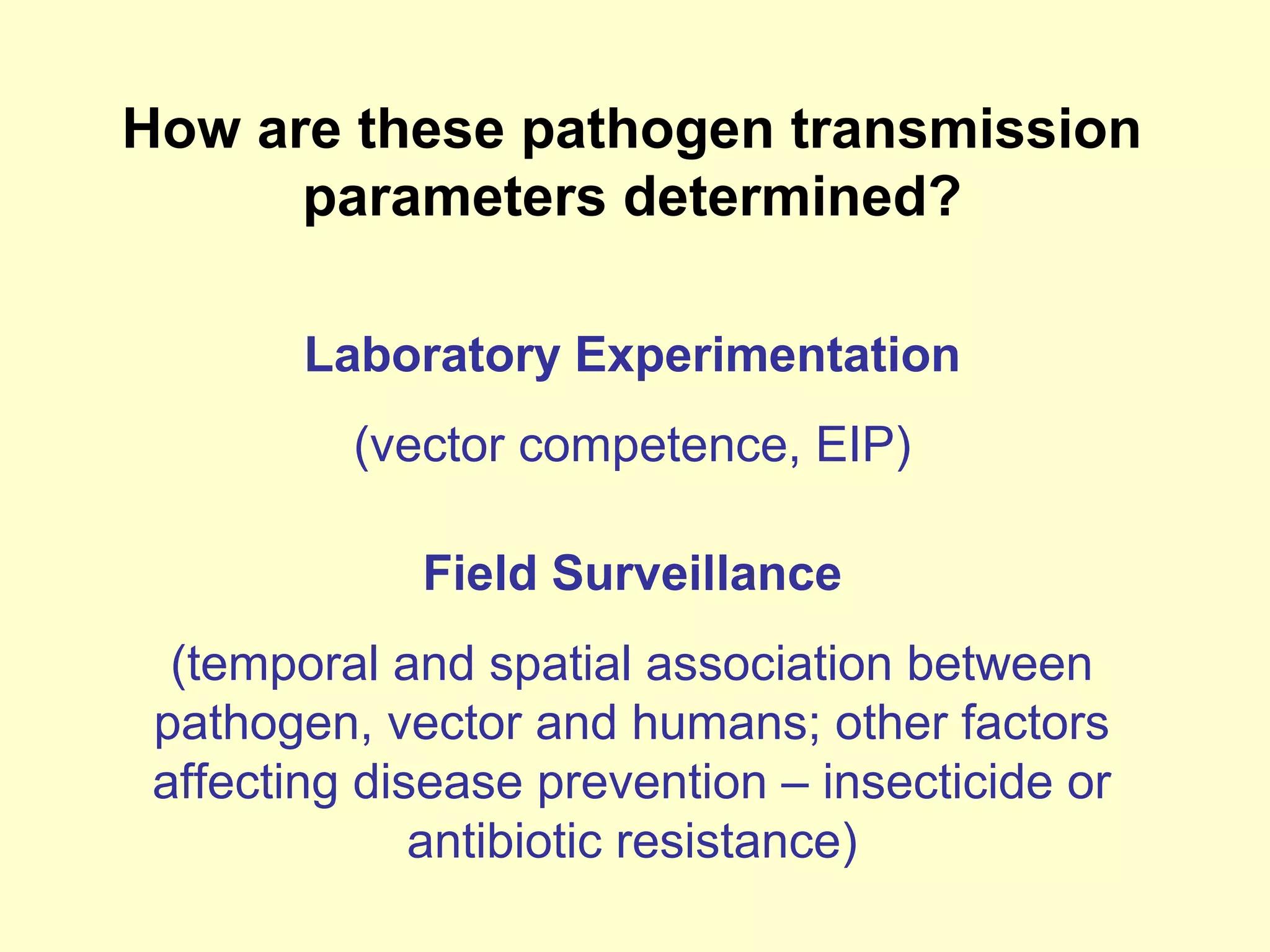
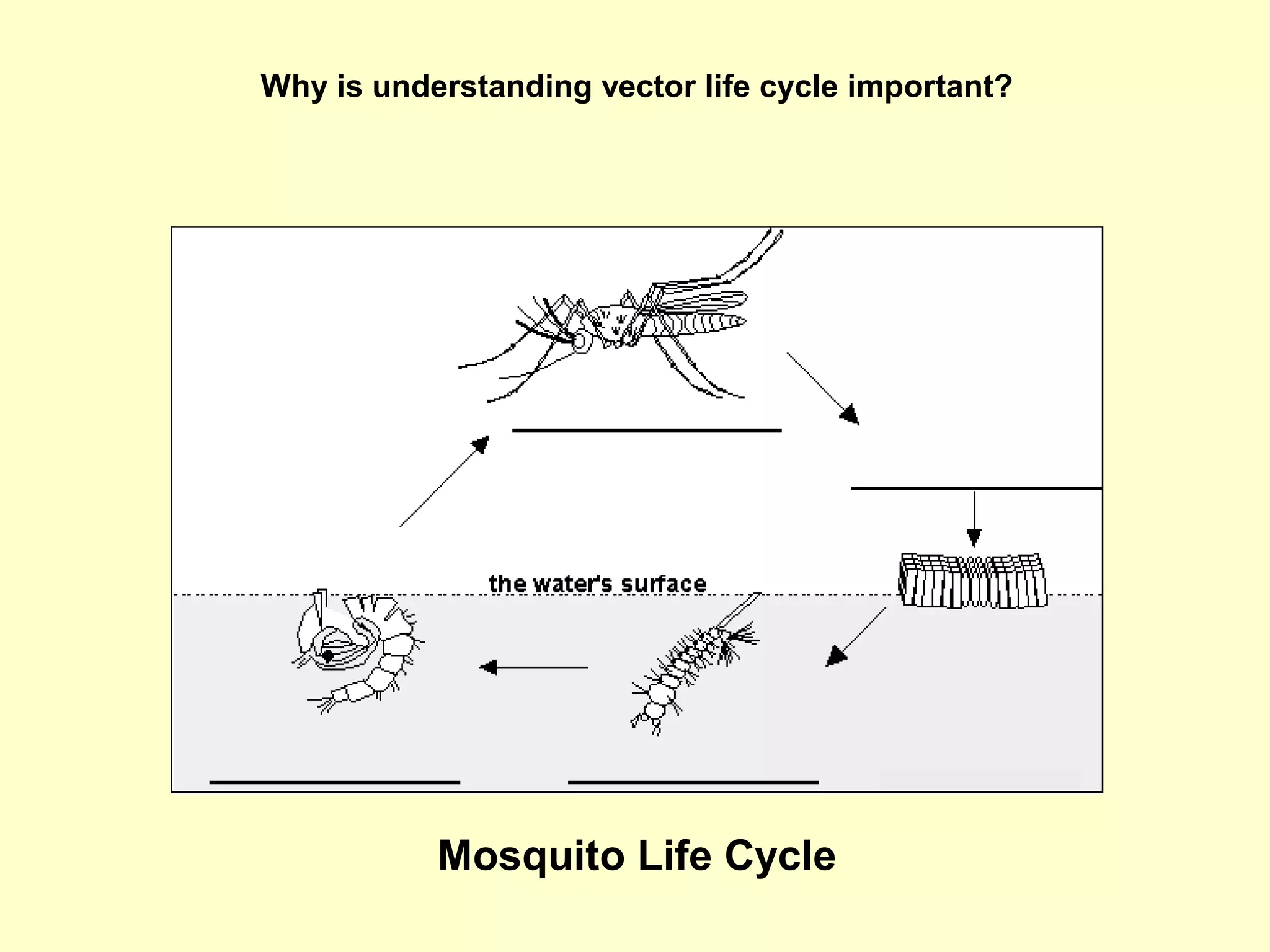
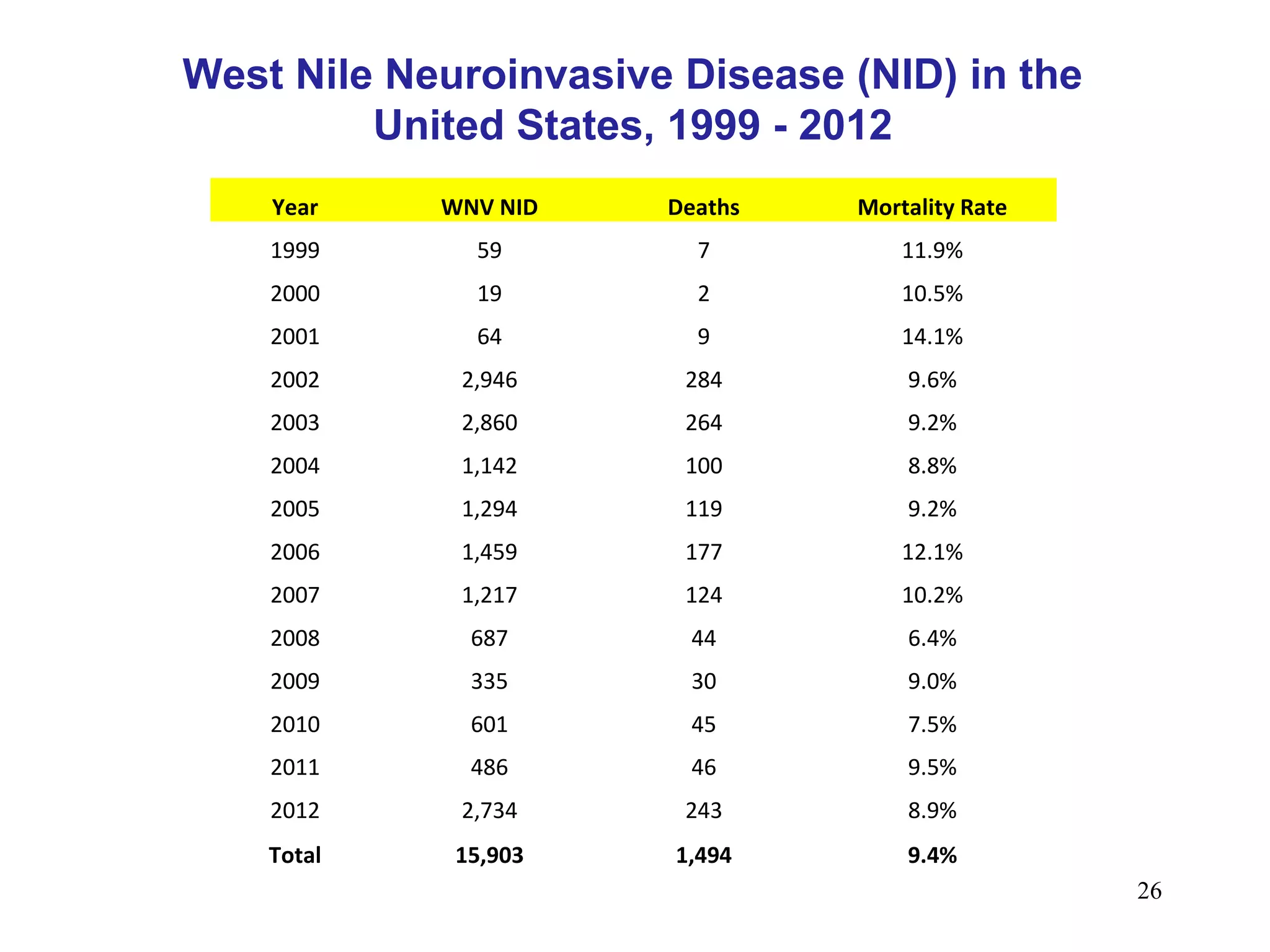
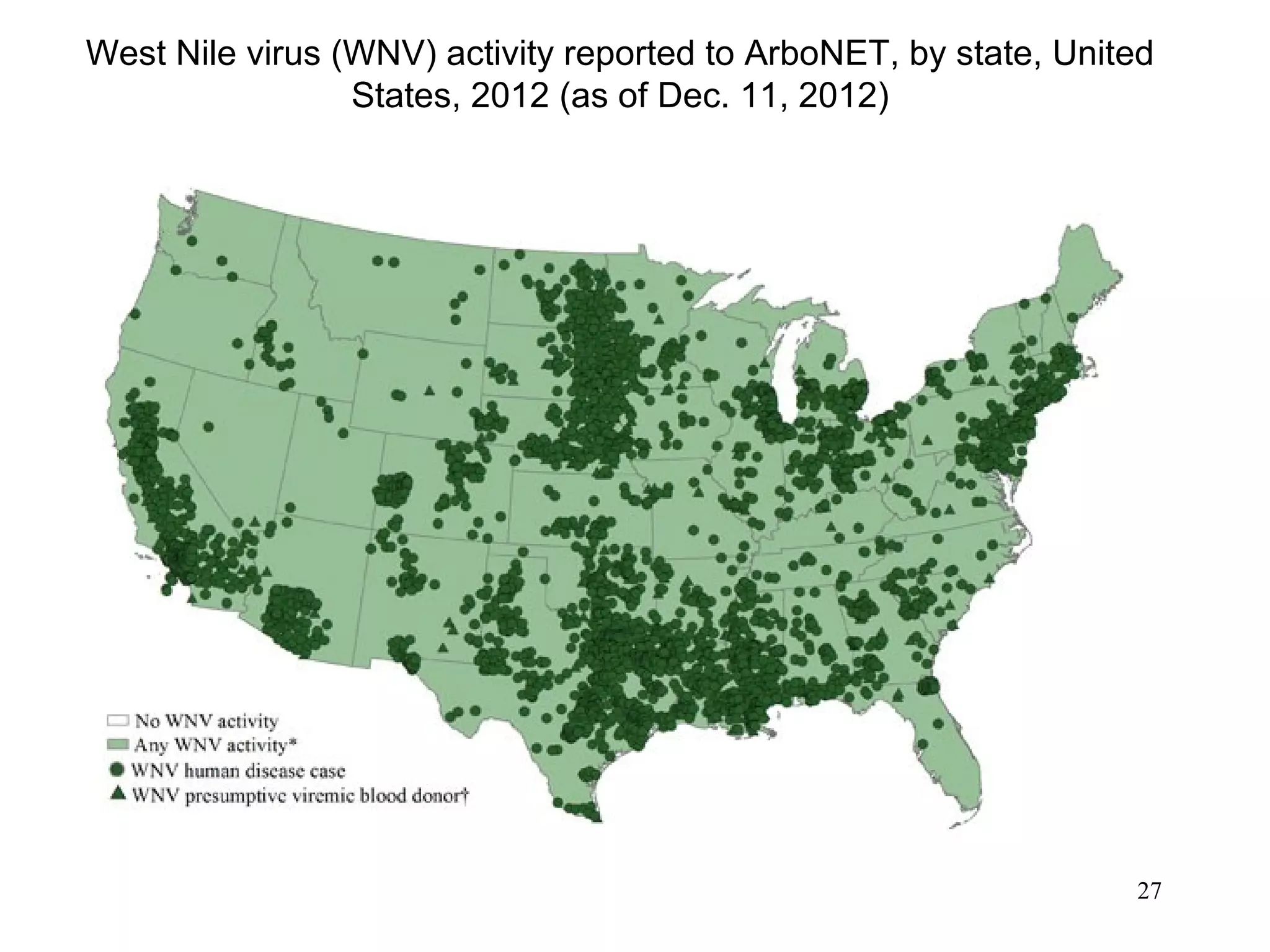
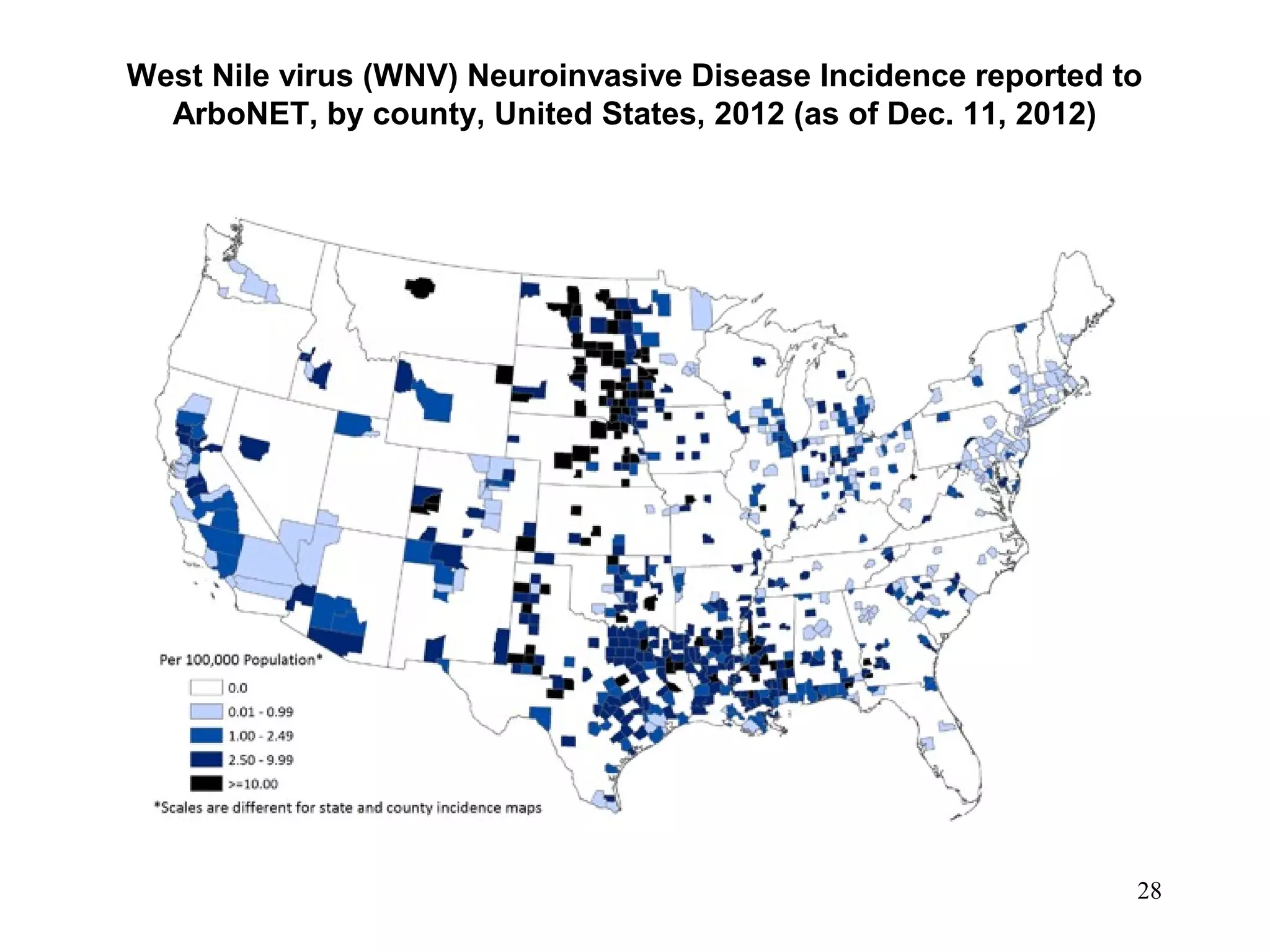
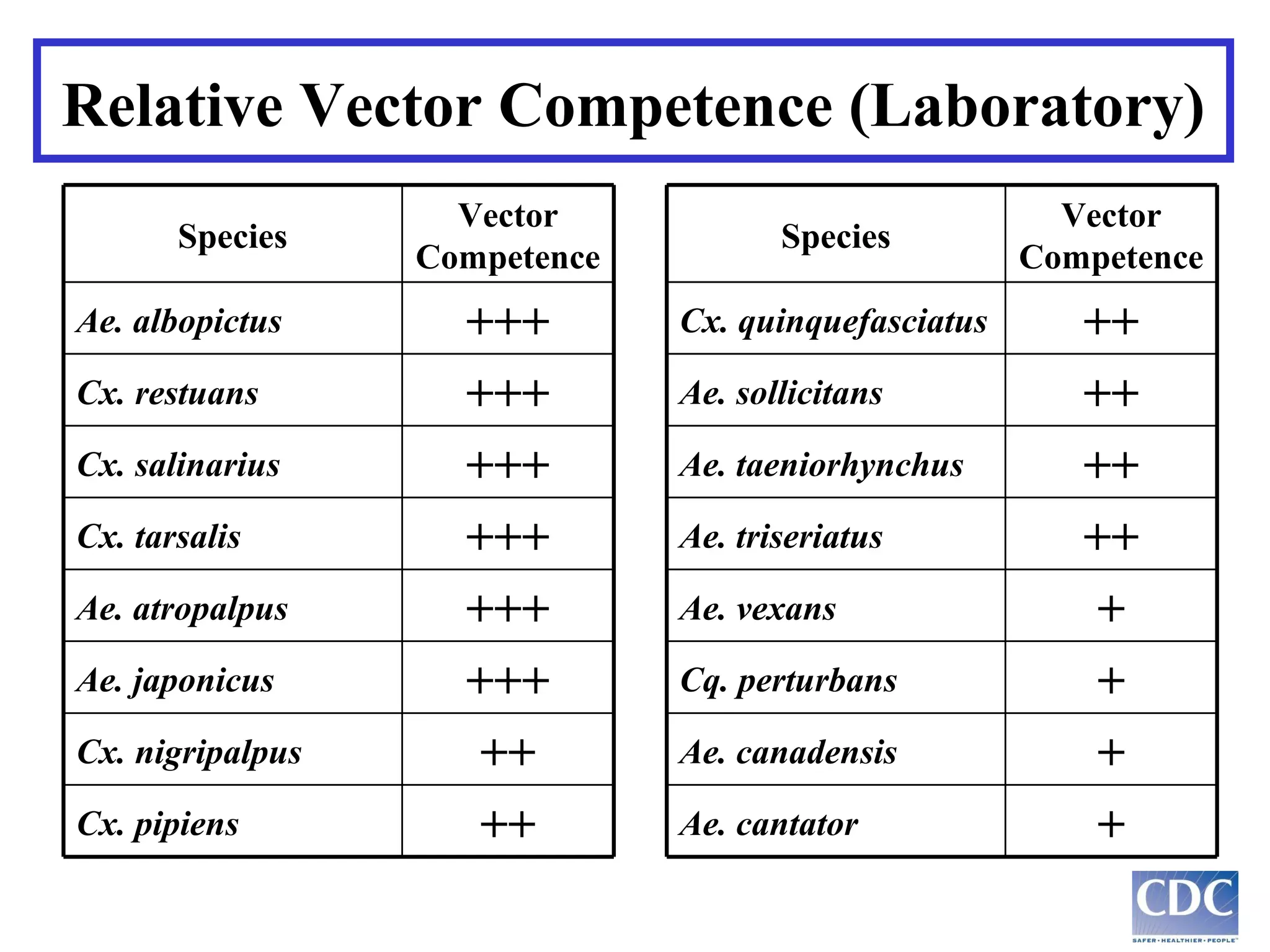
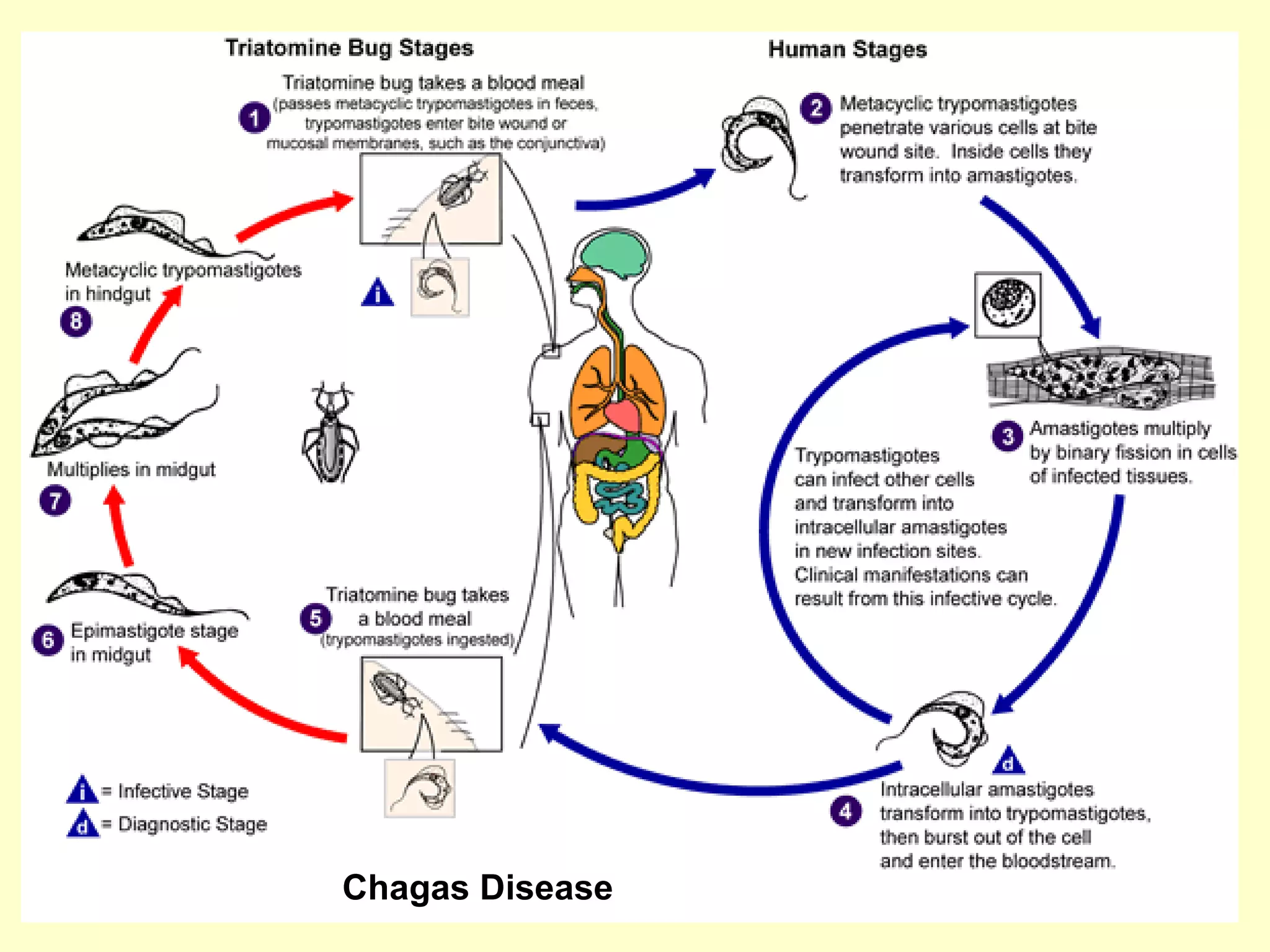
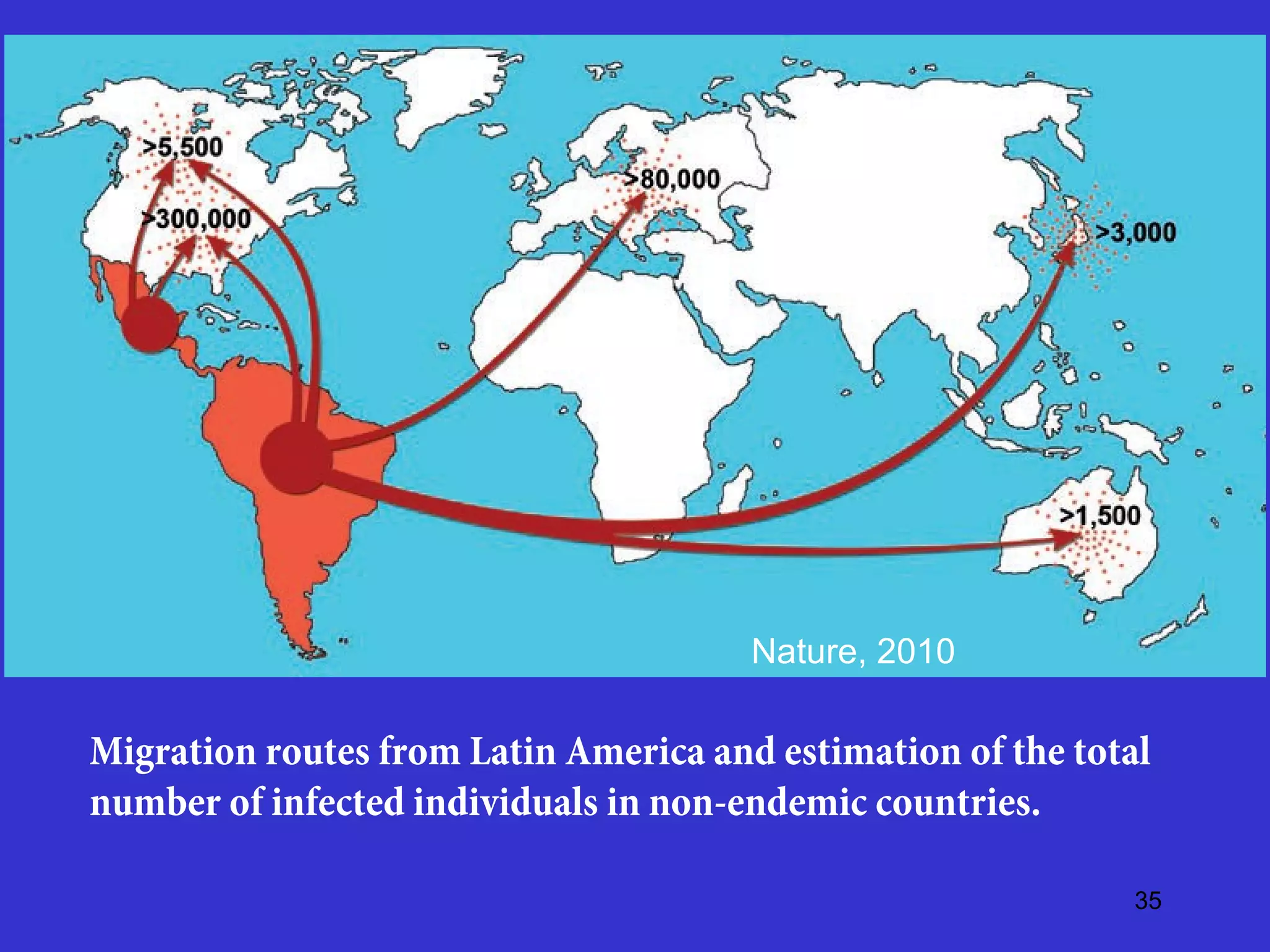
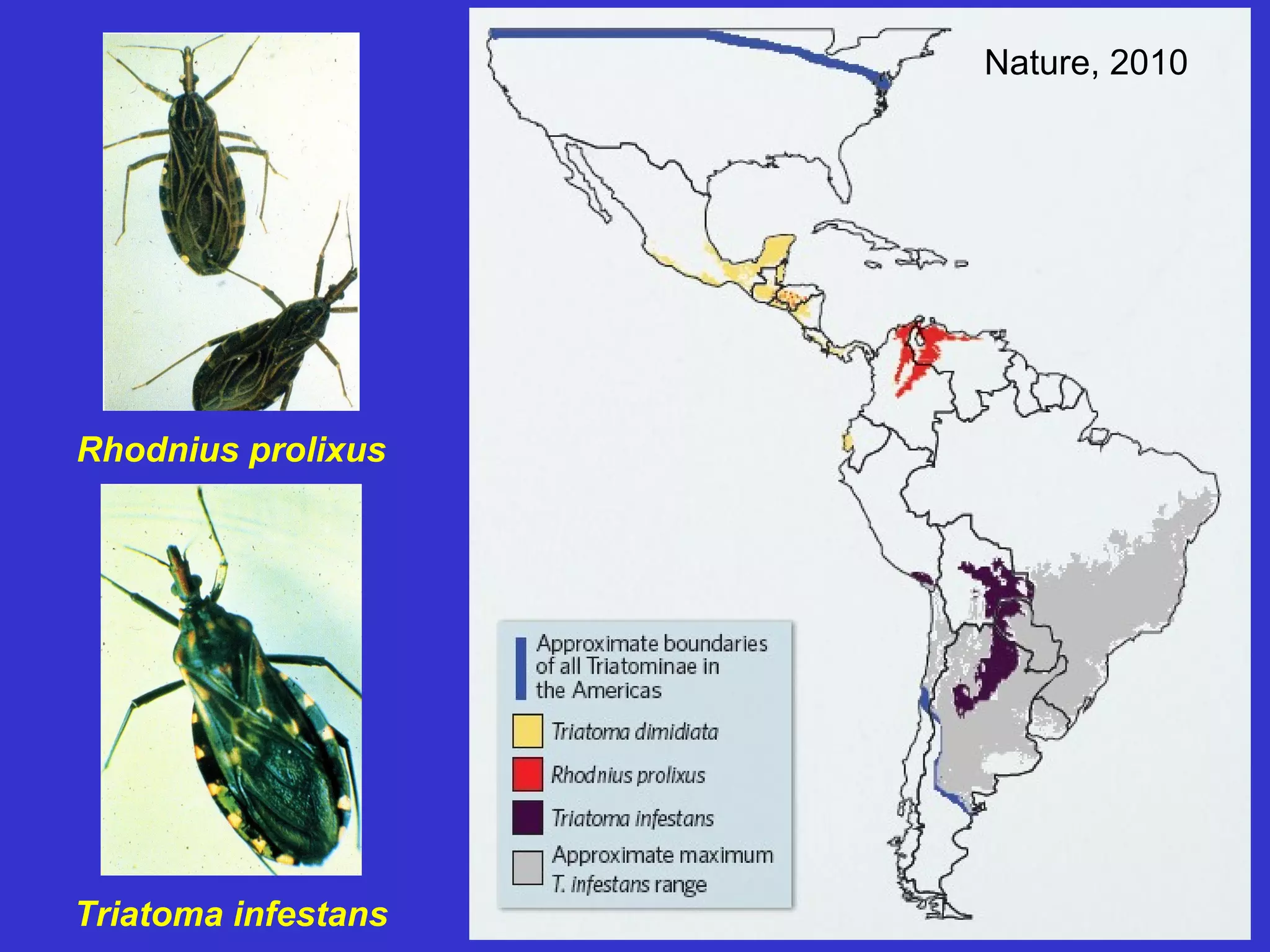
This document provides an overview of vector-borne disease (VBD) research at Tulane University, including an introduction to relevant concepts and specific disease transmission cycles. It discusses training opportunities in VBD research and Tulane's focus on diseases like dengue, West Nile virus, malaria, and Chagas disease. Key sections define vectors and types of transmission, describe important vector species and the diseases they transmit, and explain parameters important to understanding transmission like vector competence and extrinsic incubation period. Case studies of dengue, West Nile virus, and Chagas disease transmission are also presented.