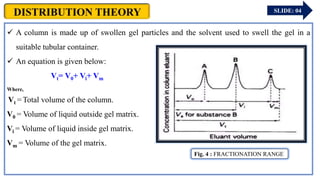
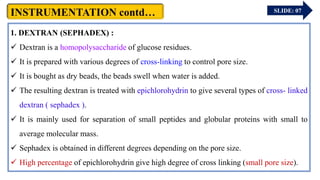
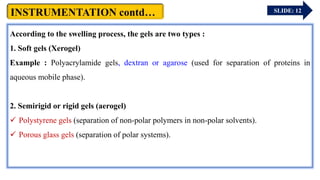
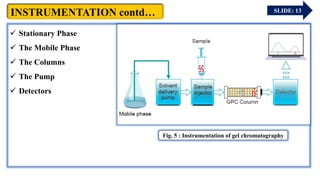
The document provides an overview of gel chromatography, a technique used for separating dissolved molecules based on size through specialized columns filled with a microporous gel. It covers fundamental principles, instrumentation, methodologies, advantages, disadvantages, and applications in purifying biological molecules. Key points include the types of gels used, separation procedures, and factors affecting elution times relative to molecular weight.