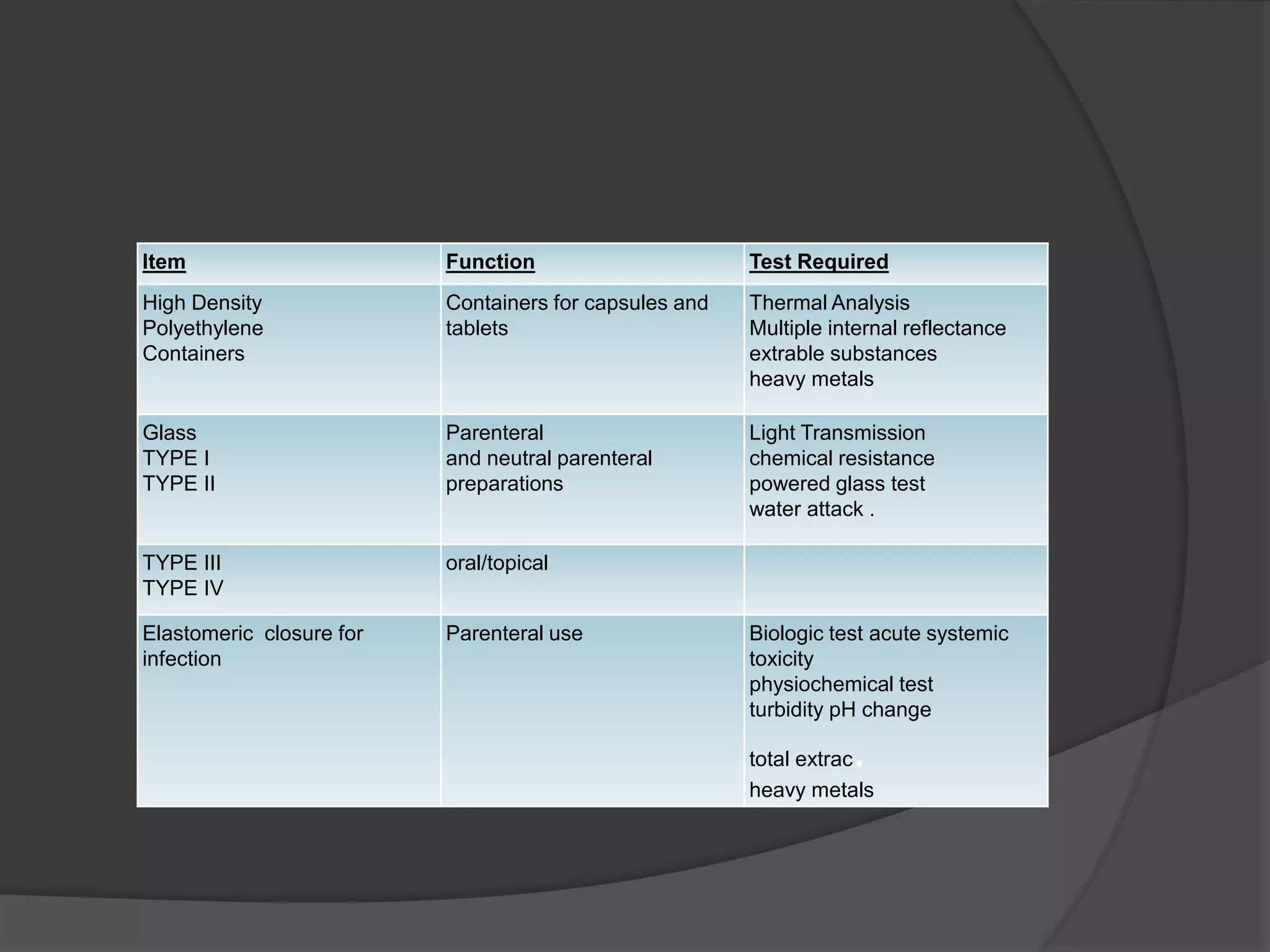
The document discusses various guidelines issued by the US Food and Drug Administration (FDA) regarding products regulated by the FDA, such as foods, drugs, medical devices, biologics, cosmetics, and radiation emitting products. It also provides details on FDA guidelines for toxicological studies, clinical trials, manufacturing sterile products, MRI safety, transport temperature maintenance, and clinical trial phases. Guidelines are presented for topics like food packaging, color additives, and maintaining quality control and assurance in accordance with FDA standards.