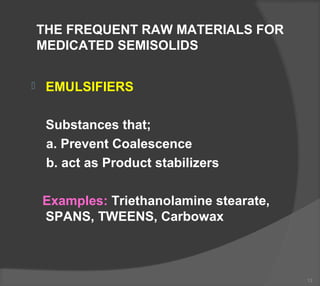
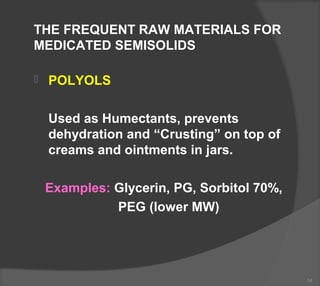
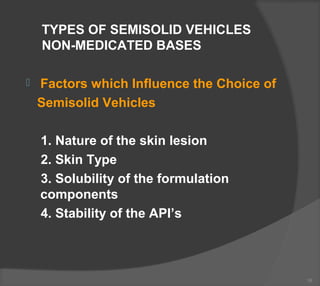
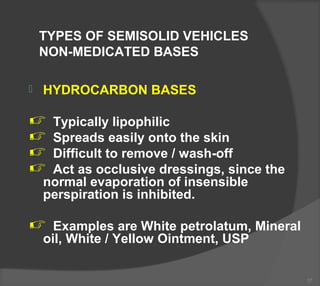
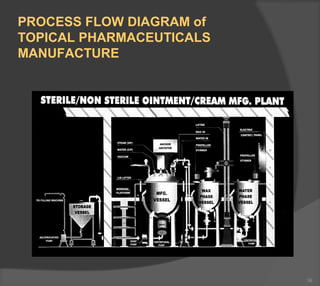
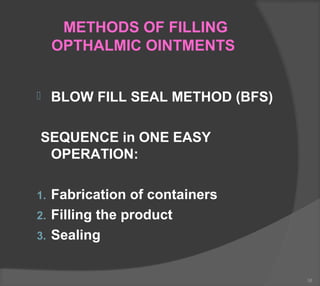
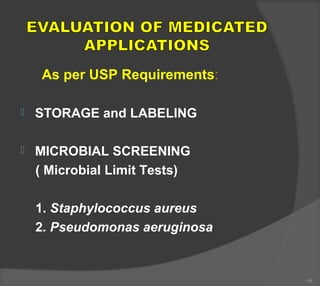
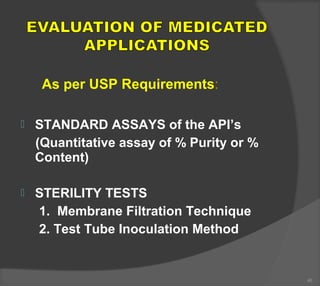
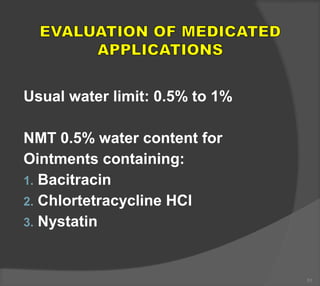
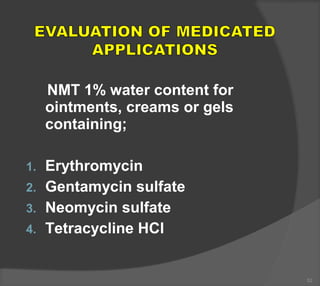
The document discusses the formulation and manufacturing processes of topical pharmaceuticals, focusing on components such as vehicles, emollients, and absorption factors affecting drug delivery through the skin. It details various raw materials used in medicated semisolid formulations, their functions, and types, alongside preservation methods and the requirements for microbiological testing. Additionally, it outlines specific manufacturing methods, especially the fusion method for semisolids, and emphasizes the importance of adhering to USP standards for quality and safety.