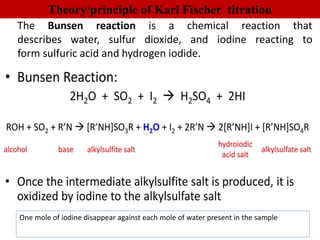
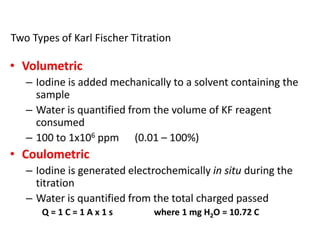
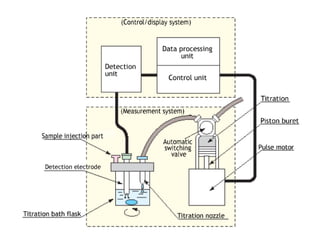
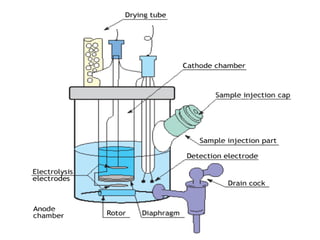
Karl Fischer titration is a technique used to determine the water content in a sample. It works based on a reaction between water, sulfur dioxide, iodine, and an alcohol solvent. The endpoint is detected electrometrically. Some advantages are its high accuracy, ability to measure small amounts of water, and suitability for automation. It has a variety of applications in industries like plastics, pharmaceuticals, chemicals and more.