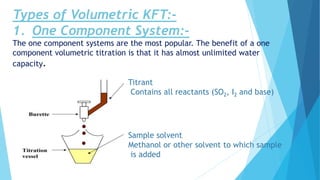
The document discusses Karl Fischer titration, a technique used to determine the water content of samples. It describes the Karl Fischer principle, where water reacts with iodine. There are two main types - volumetric titration, where iodine is added via burette, and coulometric titration, where iodine is electrochemically generated. The document outlines the Karl Fischer reaction and factors that influence it like pH. It also covers applications in industries like chemicals and pharmaceuticals and limitations of the technique.