Embed presentation


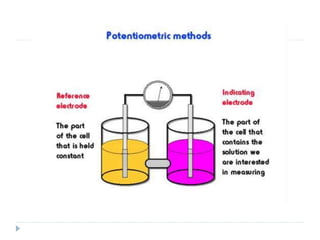

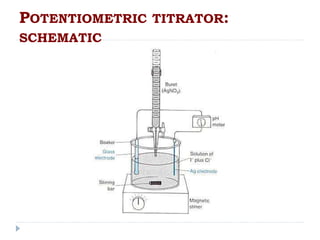
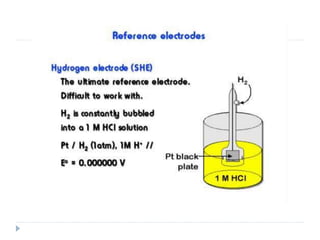
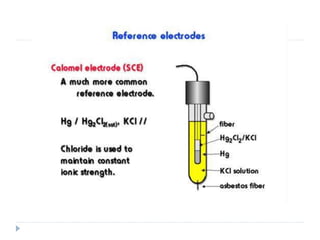

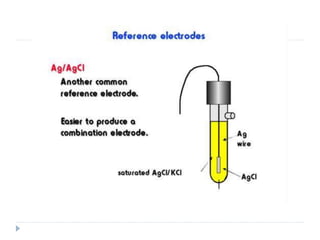
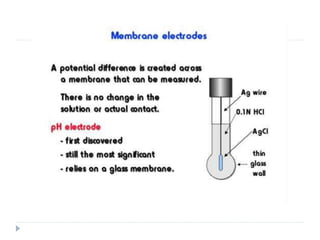
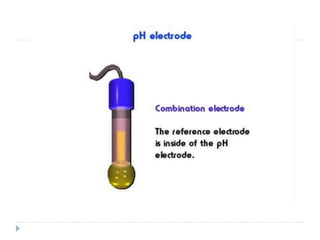

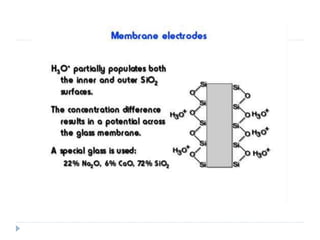

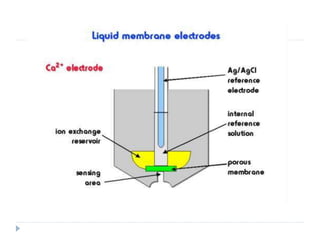

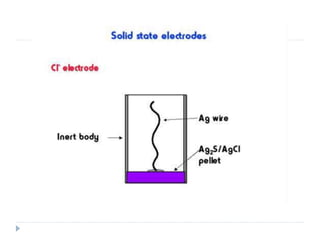

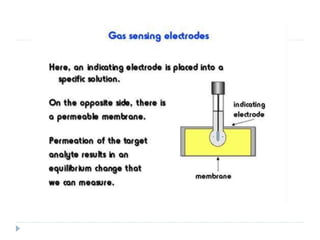


The document discusses potentiometric titration, a technique that measures voltage across an analyte without using indicators. It involves two electrodes and plots voltage against the volume of titrant added to determine the endpoint of the reaction. The document also outlines different types of potentiometric titrations including acid-base, redox, and precipitation titrations.






















