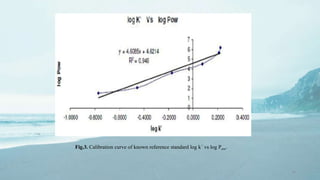
1. The document discusses methods for determining pKa (acid dissociation constant) and log P (partition coefficient) of compounds. Common methods for pKa include potentiometric titration, spectrophotometry, and NMR titration. Methods for measuring log P include shake flask experiments and HPLC.
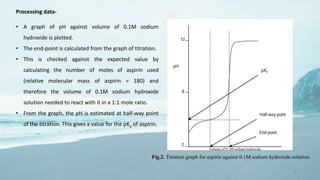
2. The document then gives an example procedure for determining the pKa of aspirin via titration with sodium hydroxide solution.
3. Factors that influence choice of solvents for measuring log P are discussed. Octanol is commonly used to model membrane permeability. The document lists typical log P values for some compounds and their applications in pharmacology.

![pKa (Logarithmic acid dissociation constant)
Introduction:
• Understanding the concept of ‘Ka’ before ‘pKa’
• Ka or acid dissociation constant is a quantitative measurement of the strength of an acid in solution.
• Let us consider the dissociation of the compound ‘HA’
HA ⇌ A- + H+
The Ka for this reaction will be given by:
Ka =
[A−][H+]
[HA]
• Expressing acidity in terms of Ka can be inconvenient for practical purposes, therefore, pKa is used.
• pKa can be defined as ‘the negative base-10 logarithm of acid dissociation constant (Ka) of a solution’.
pKa = -log10Ka
• Example:
The Ka constant for acetic acid is 0.0000158, but the pKa constant is 4.8, which is a simpler expression.
The smaller the pKa value, the stronger the acid.
The pKa value of lactic acid is about 3.8, so that means lactic acid is stronger than acetic acid. 2](https://image.slidesharecdn.com/pkaandlogppresentation-181013055958/85/pKa-and-log-p-determination-2-320.jpg)
![3
• A weak acid has a pKa value in the approximate range of -2 to 12 in water.
• Acids with a pKa value of less than about -2 are said to be strong acids.
Methods of pKa determination:
1. Potentiometric titration-
• In potentiometric titration, a sample is titrated with acid
or base using a pH electrode to monitor the course of
titration.
• The pKa value is calculated from the change in shape of
the titration curve compared with that of blank titration
without a sample present.
• Relationship between pH and pKa:
pH = pKa+log10
[A−]
[HA]
Fig.1. Titration curve of pH vs volume of titrant.](https://image.slidesharecdn.com/pkaandlogppresentation-181013055958/85/pKa-and-log-p-determination-3-320.jpg)



![7
pKa determination for aspirin:
Requirements- Aspirin, ethanol, sodium hydroxide, pH meter.
Principle- Aspirin is a weak acid and partially ionizes in water.
HA + H2O ↔ H3O+ + A-
It’s acid dissociation constant, Ka is given by:
Ka =
[A−][H3O+]
[HA]
Aspirin and sodium hydroxide react in a 1:1 mole ratio:
CH3OCOC6H4COOH + NaOH → CH3OCOC6H4COONa + H2O
Method-
• A burette is filled with 0.1M sodium hydroxide solution.
• 0.36g of aspirin is weighed in 250ml beaker and 10ml of 95% ethanol is added and volume is made up with deionized water.
• 2ml portions of sodium hydroxide solution is added from burette to the beaker, stirring well between each additions and recording
the pH using a pH meter.
• The pH begins to rise rapidly near the end-point.
• After adding 18ml of sodium hydroxide solution, addition is continued in 0.5ml portions.
• After adding about 22ml, additions in 2ml portions is started again.
• The addition is continued until total of 36ml has been added.](https://image.slidesharecdn.com/pkaandlogppresentation-181013055958/85/pKa-and-log-p-determination-7-320.jpg)
![9
Log P (Logarithm of partition-coefficient)
Introduction:
• Partition coefficient, abbreviated as P, is defined as a particular ratio of the concentrations of a solute between two immiscible
solvents at equilibrium.
• The logarithm of this ratio is thus ‘log P’.
log Pow = log
[unionized solute]o
[unionized solute]w
• This ratio is therefore a measure of the difference in solubility of the compound in these two phases.
• The partition-coefficient generally refers to the concentration ratio of unionized species of the compound.
• When one of the solvents is water and the other is a non-polar solvent, then the log P value is a measure of lipophilicity or
hydrophobicity.
Choice of solvents:
• The choice of partition solvent has been subject to debate in recent years.
• The most commonly used solvent has been octan-1-ol or octanol.
• Octanol was chosen as a simple model of a phospholipid membrane.](https://image.slidesharecdn.com/pkaandlogppresentation-181013055958/85/pKa-and-log-p-determination-9-320.jpg)