This document provides information on total anomalous pulmonary venous connection (TAPVC), including:
1. TAPVC is a congenital heart defect where the pulmonary veins do not connect normally to the left atrium and instead connect to the right atrium or its tributaries.
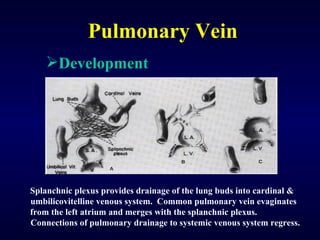
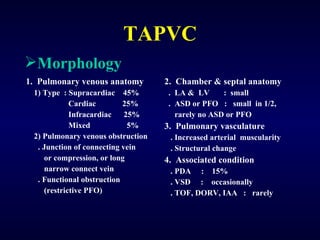
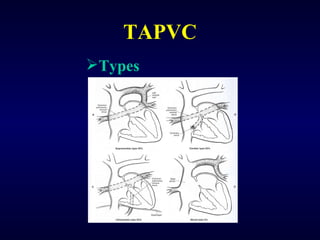
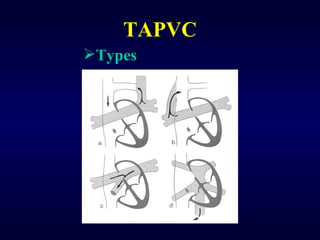
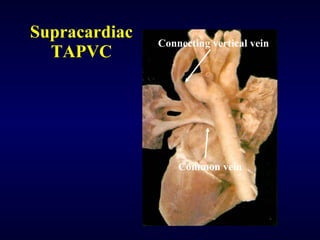
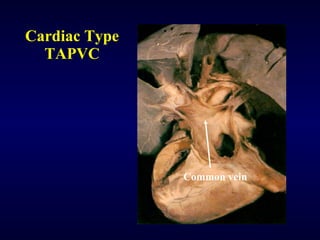
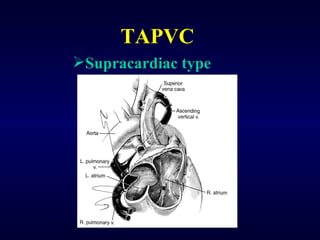
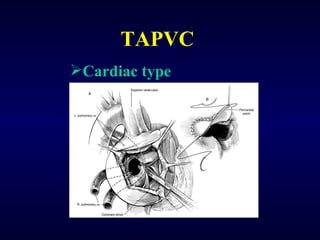
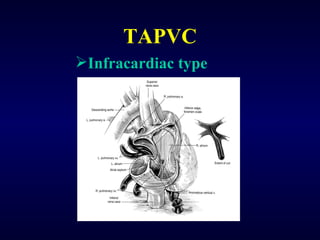
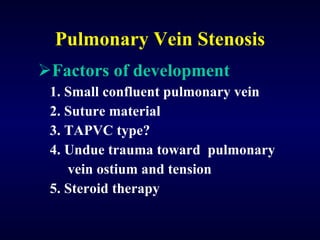
2. There are four main types of anomalous pulmonary vein connections: supracardiac, cardiac, infracardiac, and mixed.
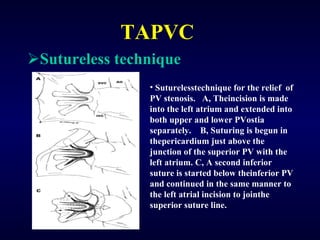
3. Untreated TAPVC can lead to pulmonary overcirculation, congestion, and pulmonary hypertension due to mixing of oxygenated and deoxygenated blood. Surgical repair is usually recommended.