Ebstein's anomaly is a rare congenital heart defect where the tricuspid valve does not form properly, causing the lower right chamber of the heart (right ventricle) to function poorly. Key features include downward displacement of the tricuspid valve into the right ventricle, an enlarged upper right chamber (right atrium), and a small, non-functional portion of the right ventricle. Presentation varies from no symptoms to heart failure in infancy. Complications include arrhythmias, paradoxical embolism, and sudden cardiac death. Diagnosis is made based on echocardiogram findings and symptoms. Treatment involves medication, surgery, or heart transplant depending on severity.















![RT Heart – 3 components
• RA Proper
• Inlet portion of RV [Atrialized]
• Trabecular and outlet portion[Functional RV]
• The greater the apical displacement of the
posterior and septal leaflets, the larger the
atrialized RV and the smaller the functional RV.](https://image.slidesharecdn.com/ebsteinsanamolynew-161211185055/85/Ebsteins-anamoly-16-320.jpg)

![Atrialized RV
• Thin walled
• Devoid of muscle tissue
• Dilated often aneurysmally[1/2 of RV volume]
• Paradoxical expansion in systole –passive
reservoir](https://image.slidesharecdn.com/ebsteinsanamolynew-161211185055/85/Ebsteins-anamoly-18-320.jpg)














![Rt to Lt shunt
• Neonates [ high PVR]
• Old age - RV Filling pressure ↑
• Stenotic/imperforate - T.orifice](https://image.slidesharecdn.com/ebsteinsanamolynew-161211185055/85/Ebsteins-anamoly-34-320.jpg)



















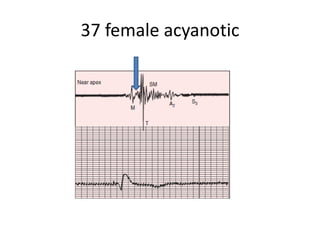
![AUSCULTATION
• S1 – Wide split
• M1 – T1 ,Loud and delayed T1[Sail sound]
• Delayed T1 – Complete RBBB, Hypokinetic RV, Large
and increased excursion and tension of ATL .
• PR prolongation – soft M1
• Preexcitation of RV – Early loud T1 /Buried M1](https://image.slidesharecdn.com/ebsteinsanamolynew-161211185055/85/Ebsteins-anamoly-54-320.jpg)