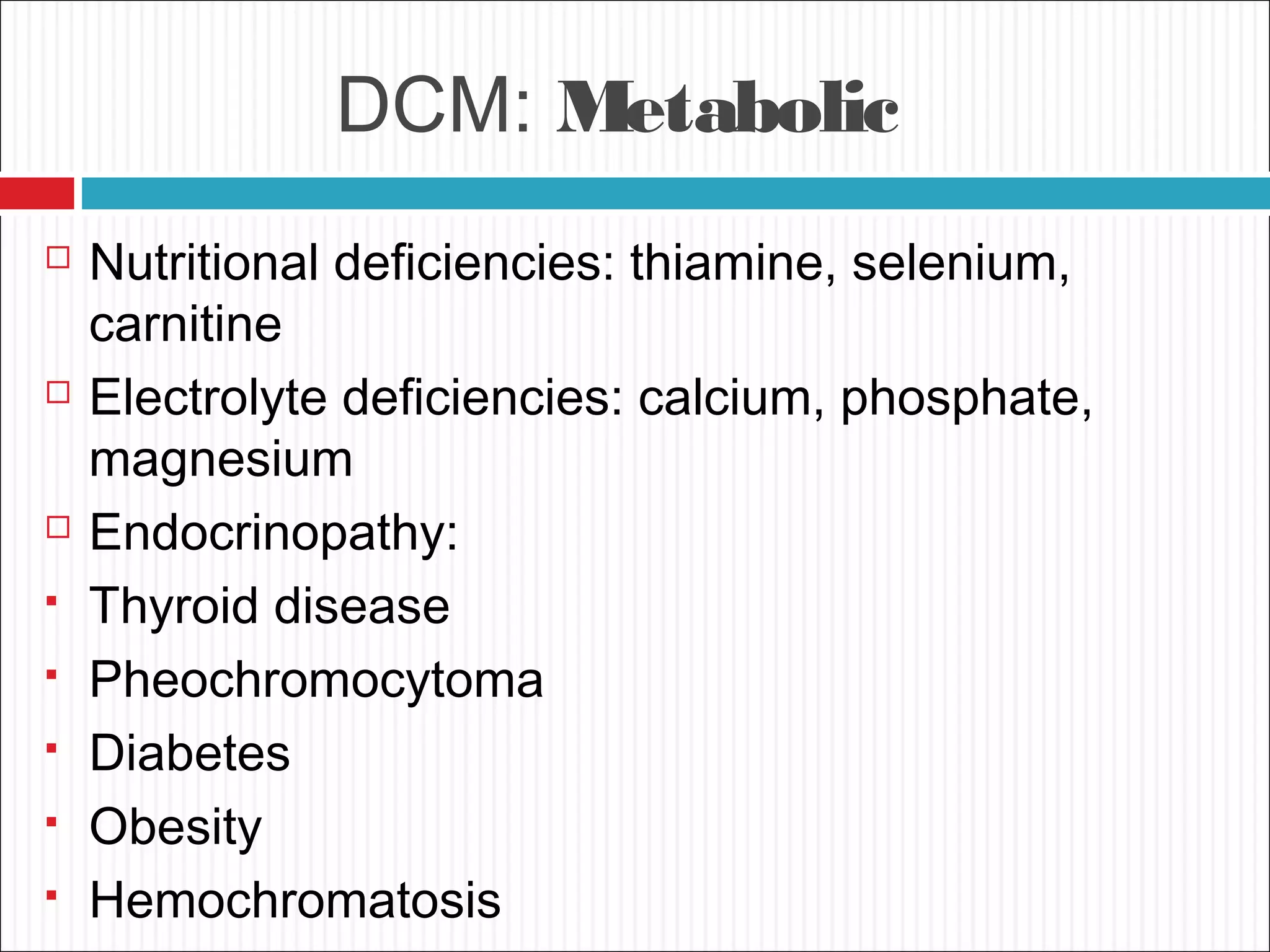
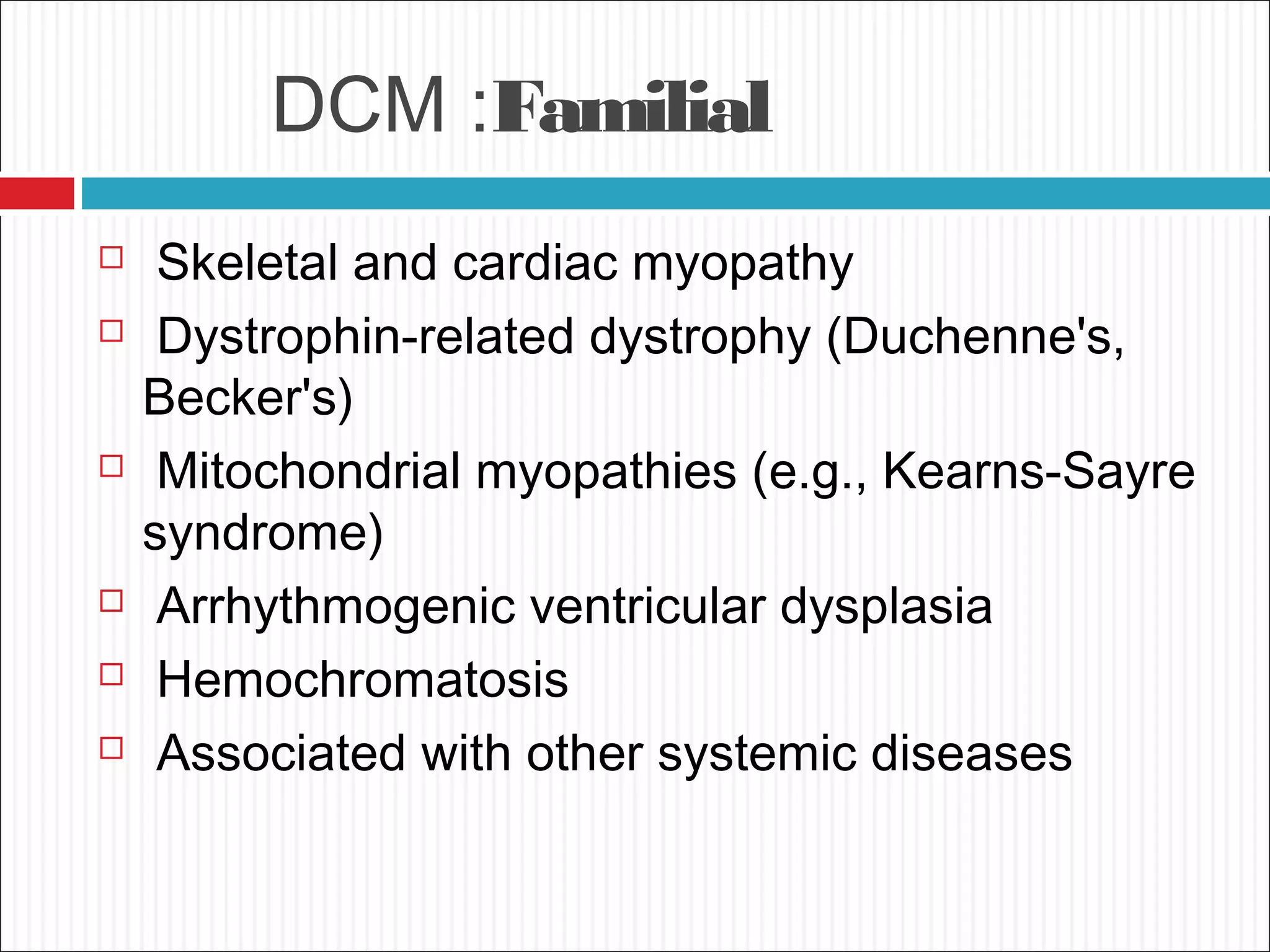
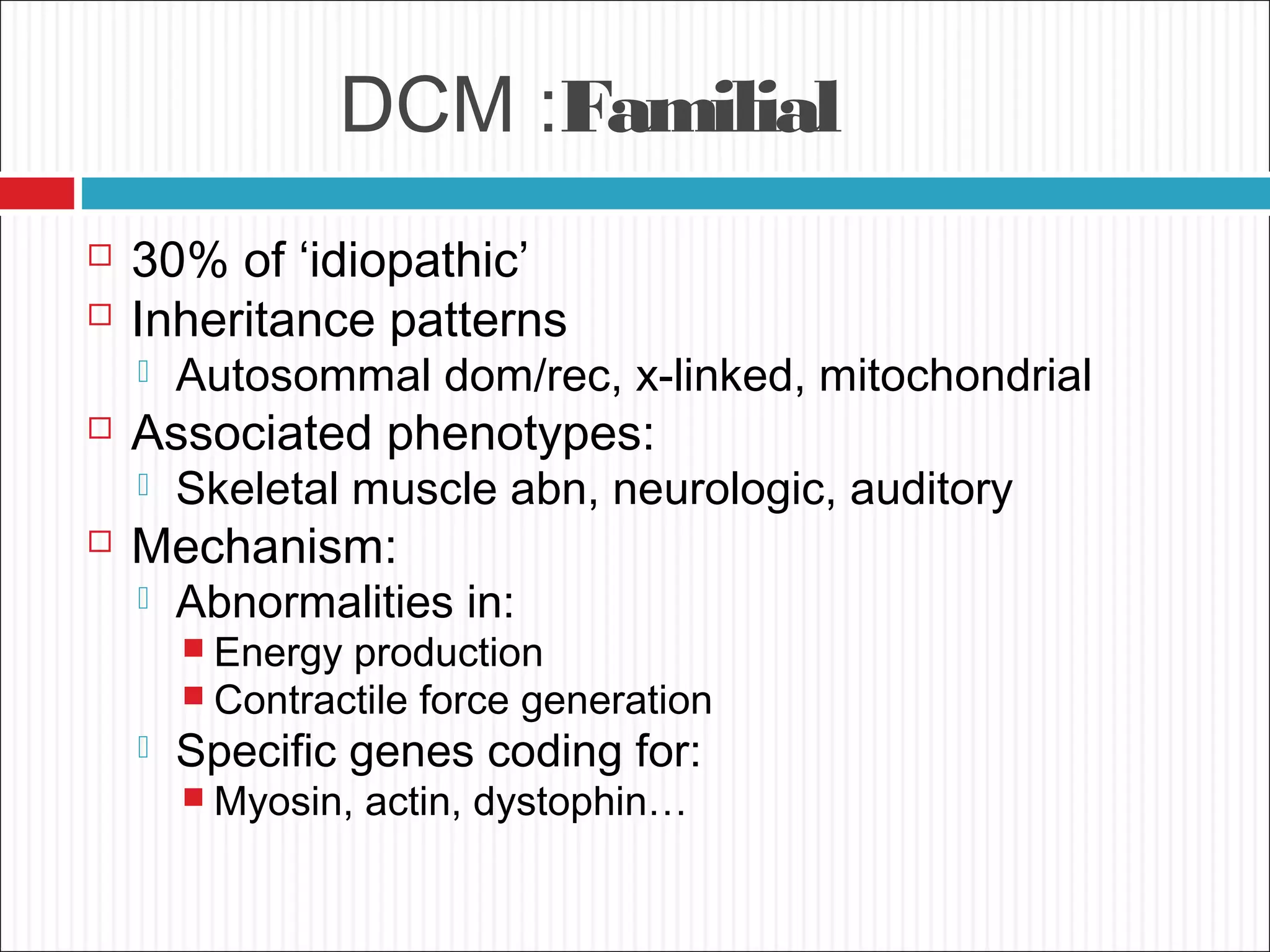
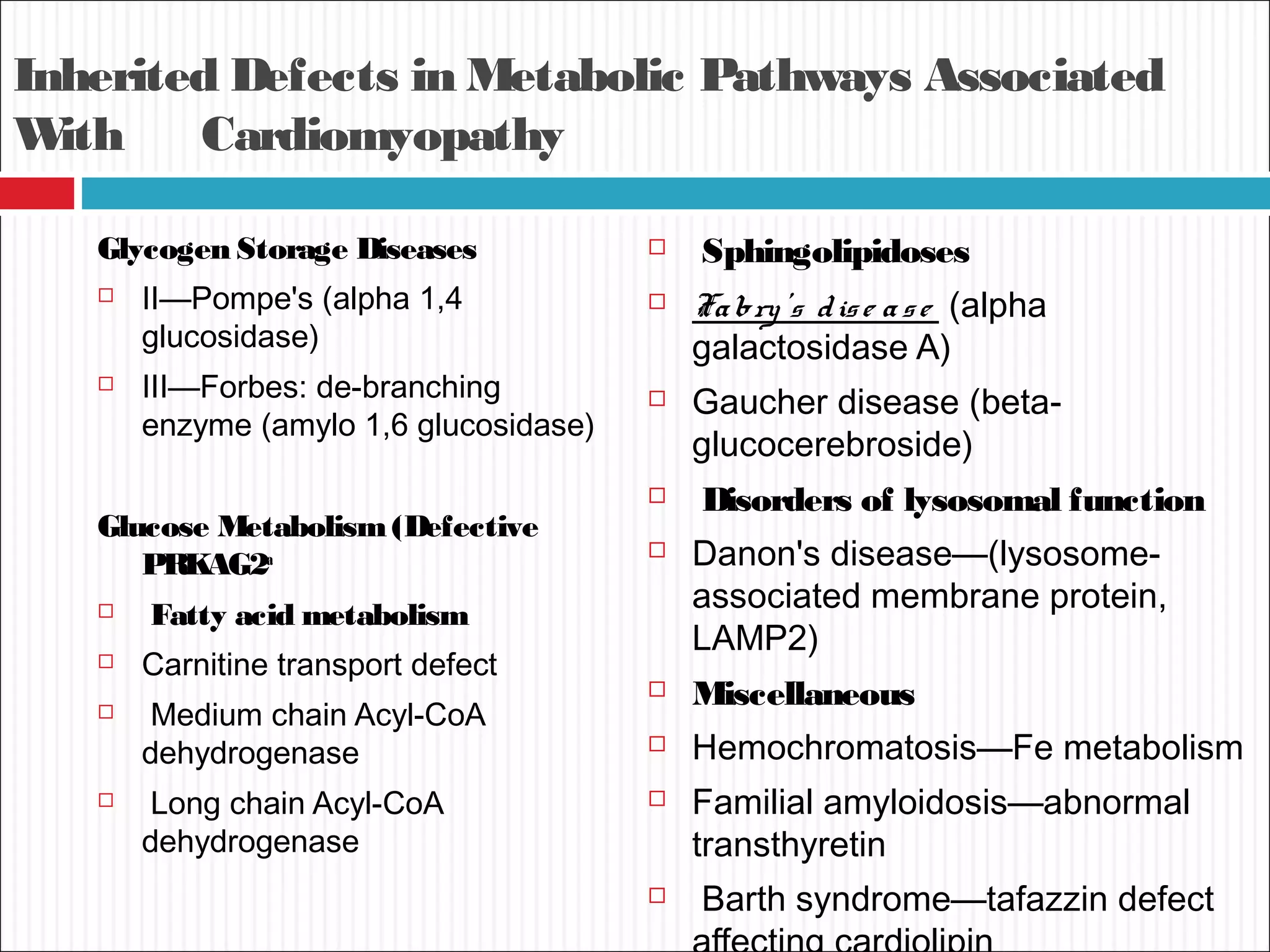
Dilated cardiomyopathy is characterized by an enlarged and poorly contracting left ventricle. The main causes include inflammatory, toxic, metabolic, inherited, idiopathic and miscellaneous factors. On evaluation, patients typically present with decreased cardiac output, tachycardia and signs of congestion. Tests include echocardiogram, which shows increased left ventricular size and reduced ejection fraction, and ECG, which may show conduction delays. Treatment focuses on managing symptoms with diuretics and blocking neurohormonal activation to prevent worsening, while devices like ICDs are used in eligible patients.