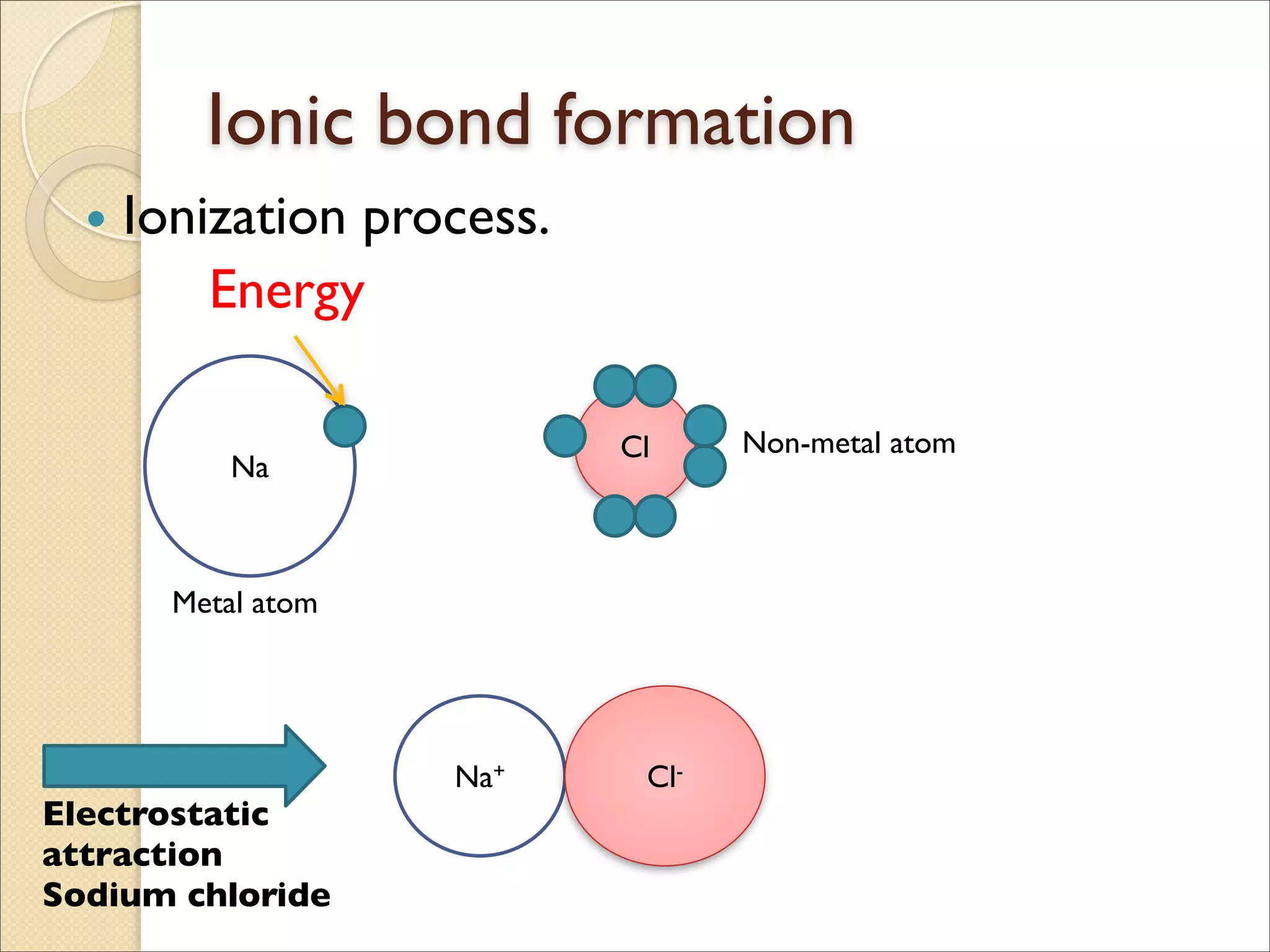
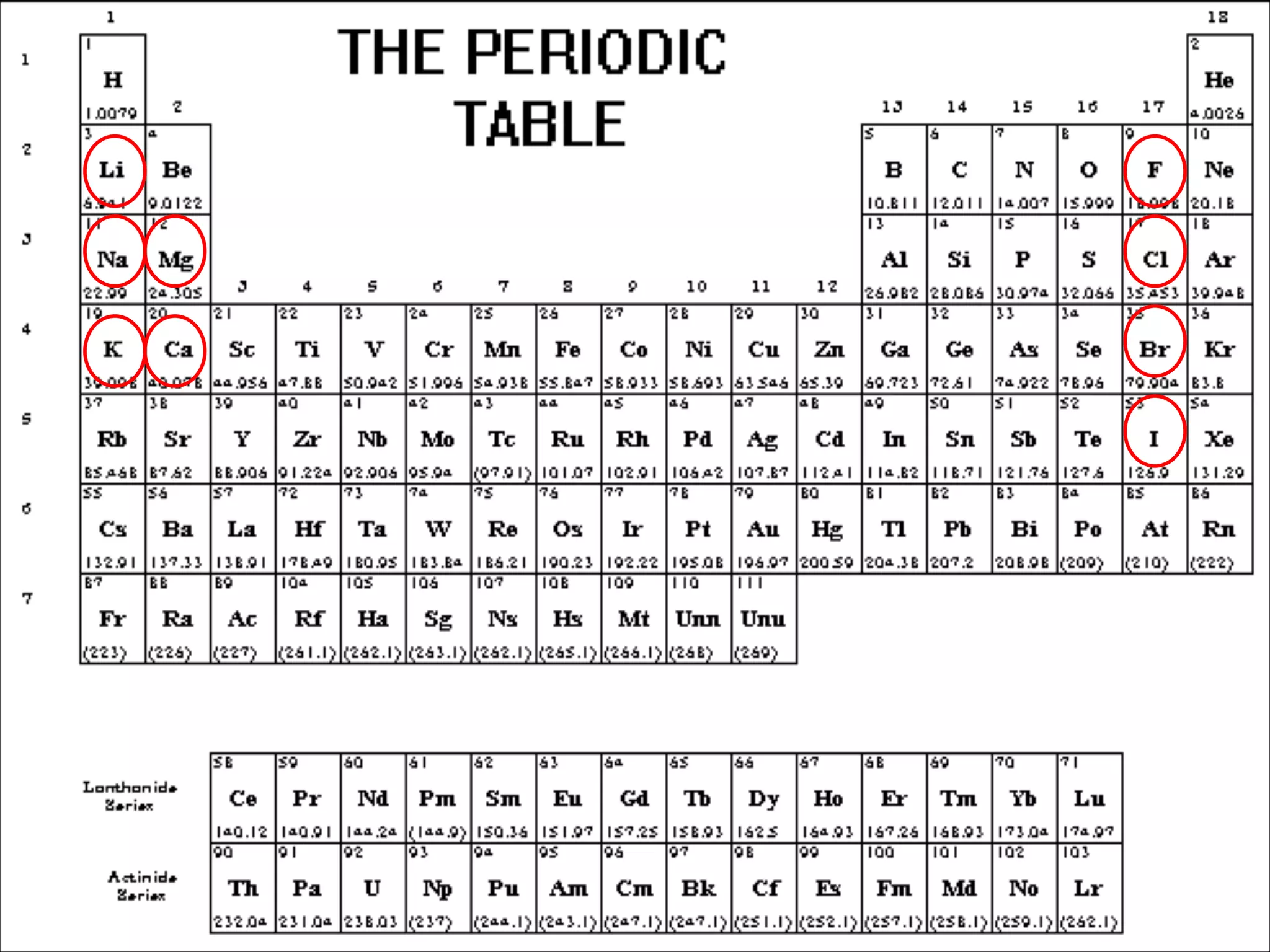
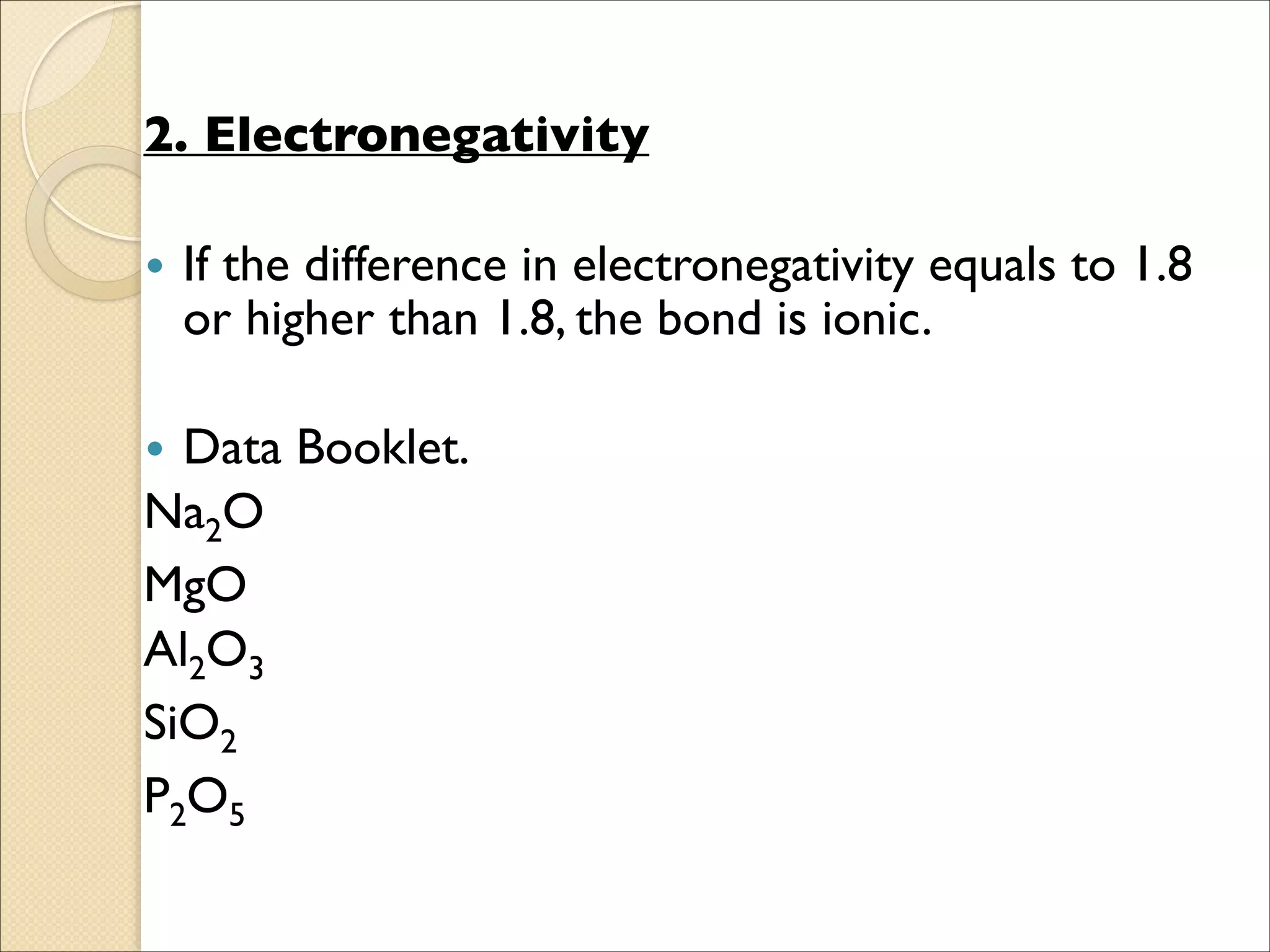
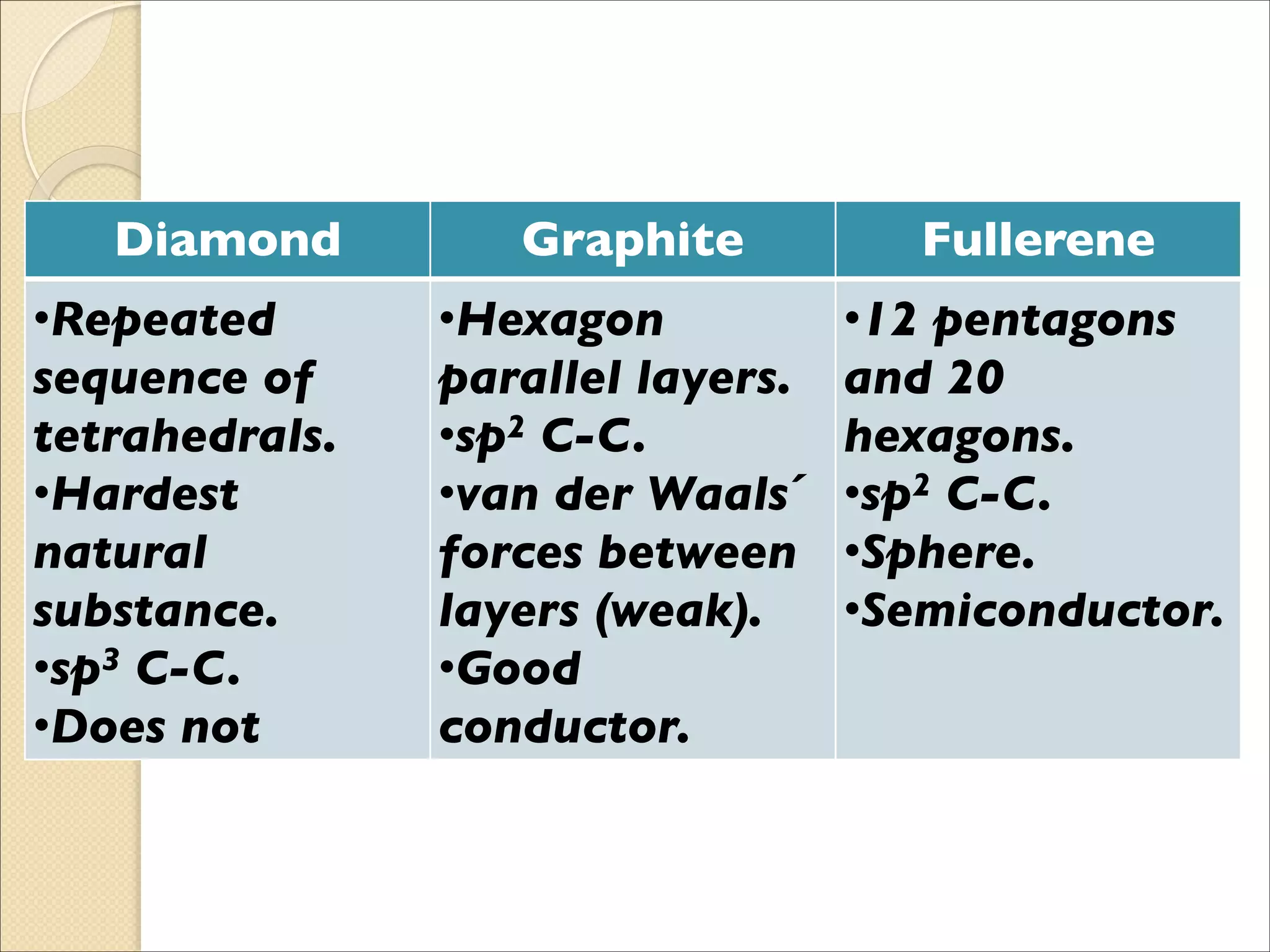
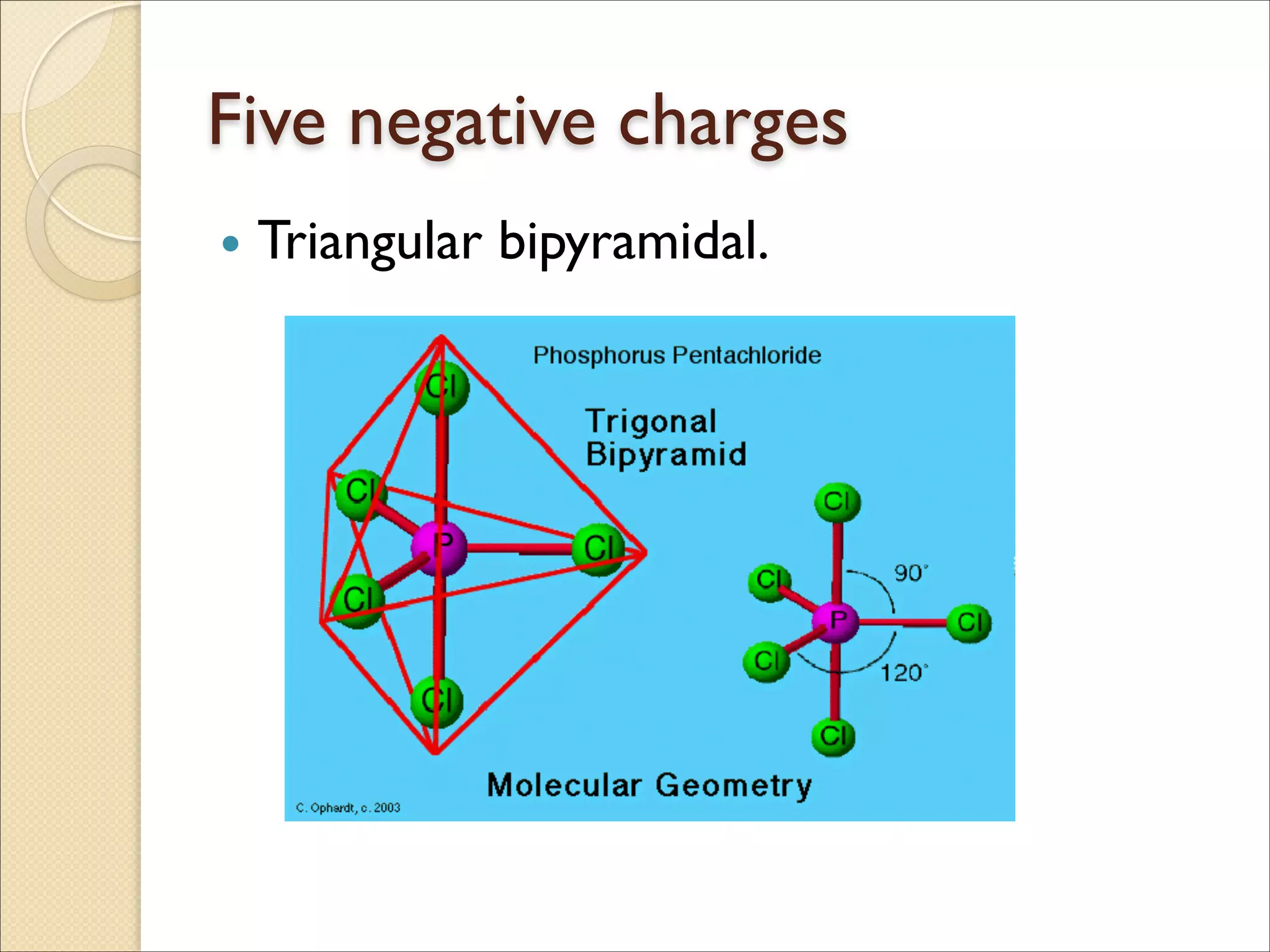
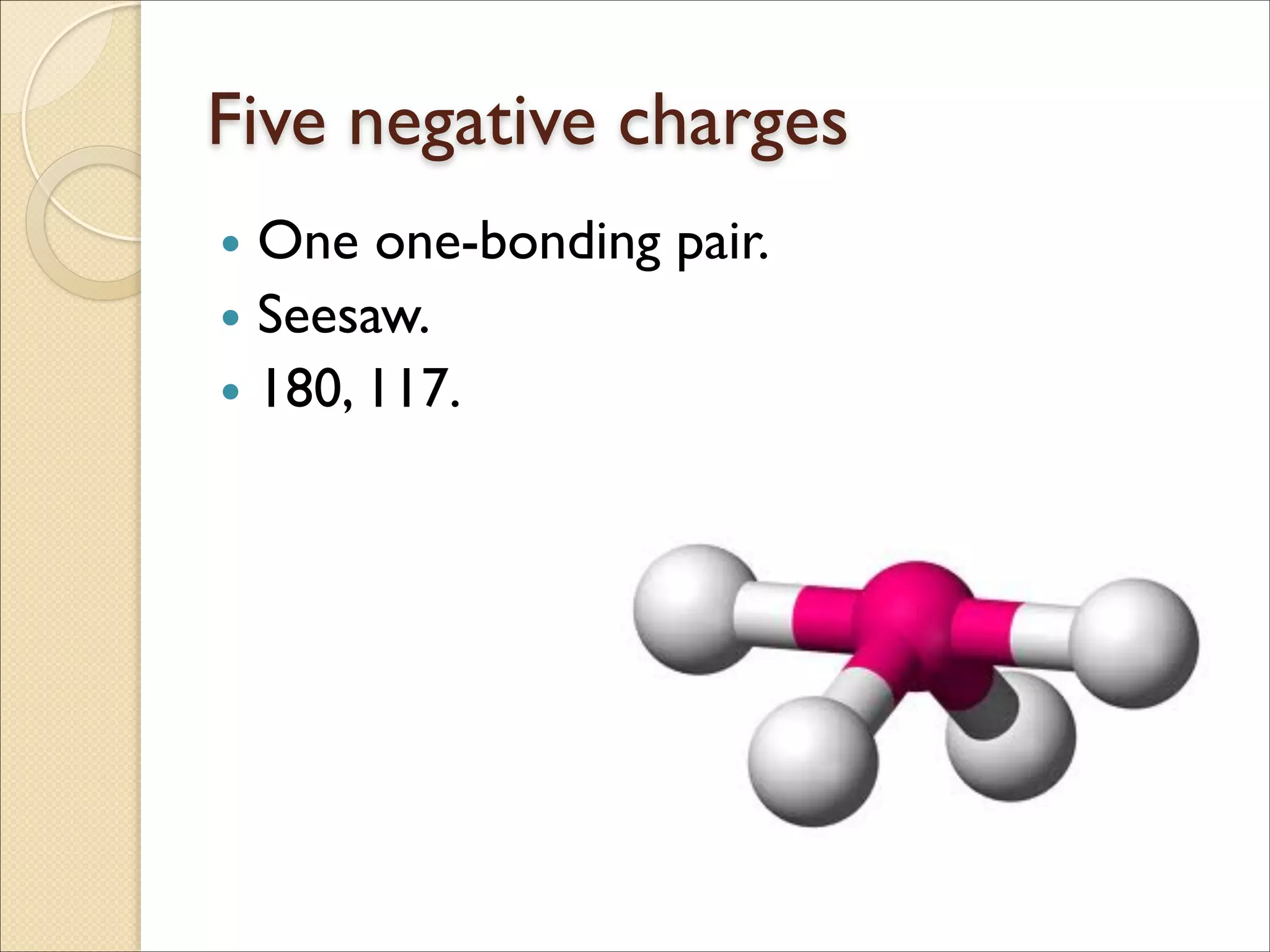
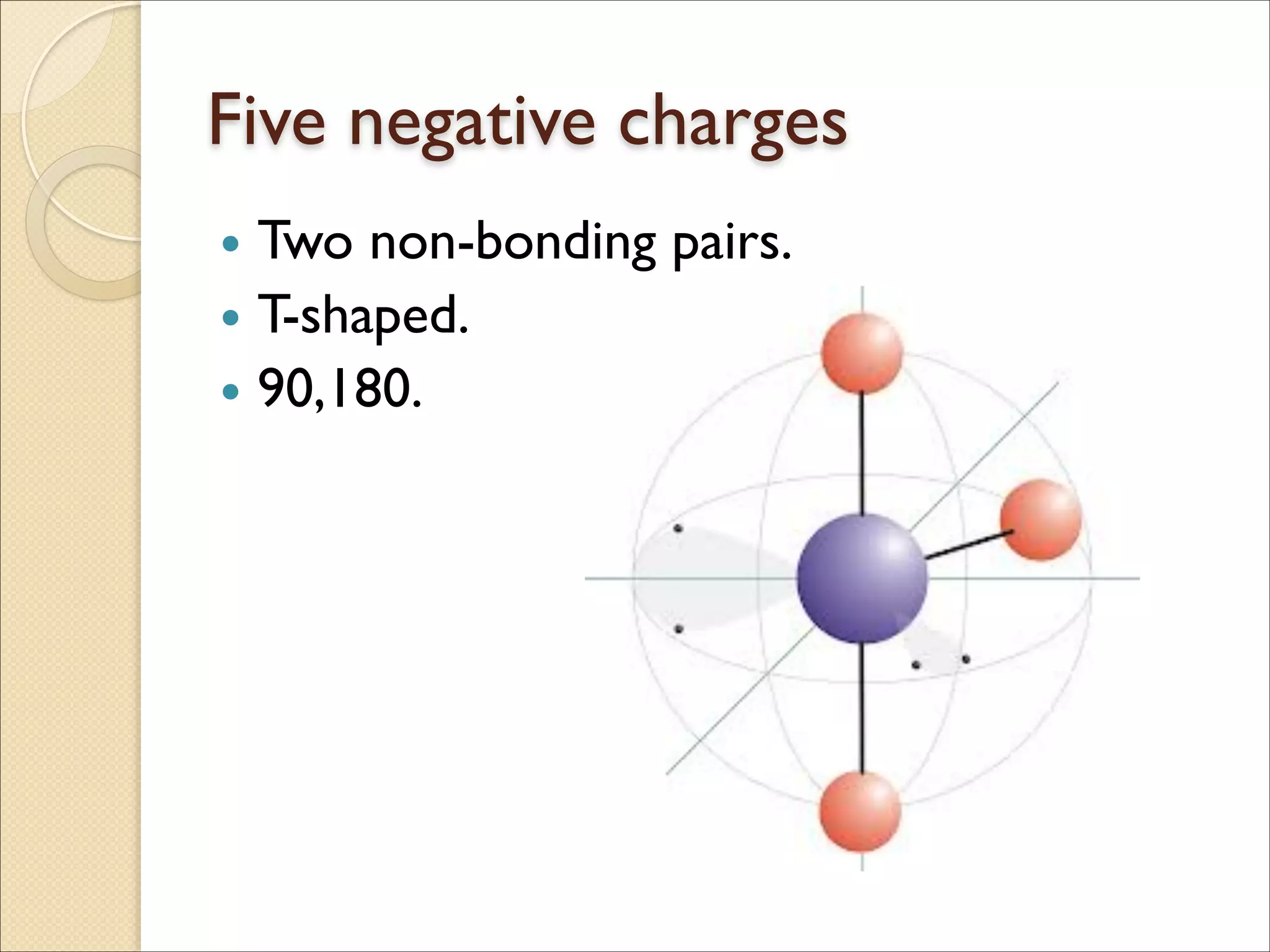
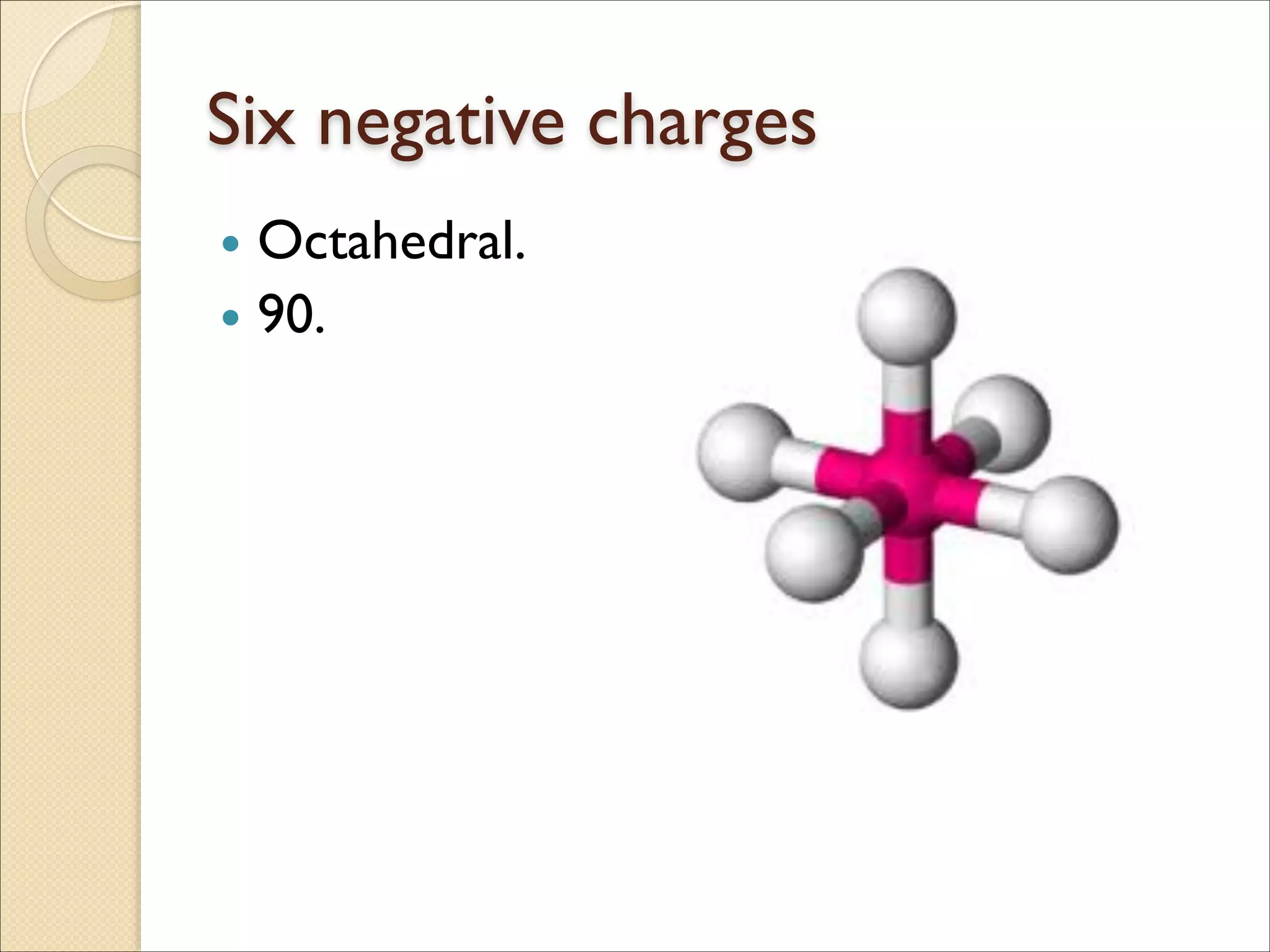
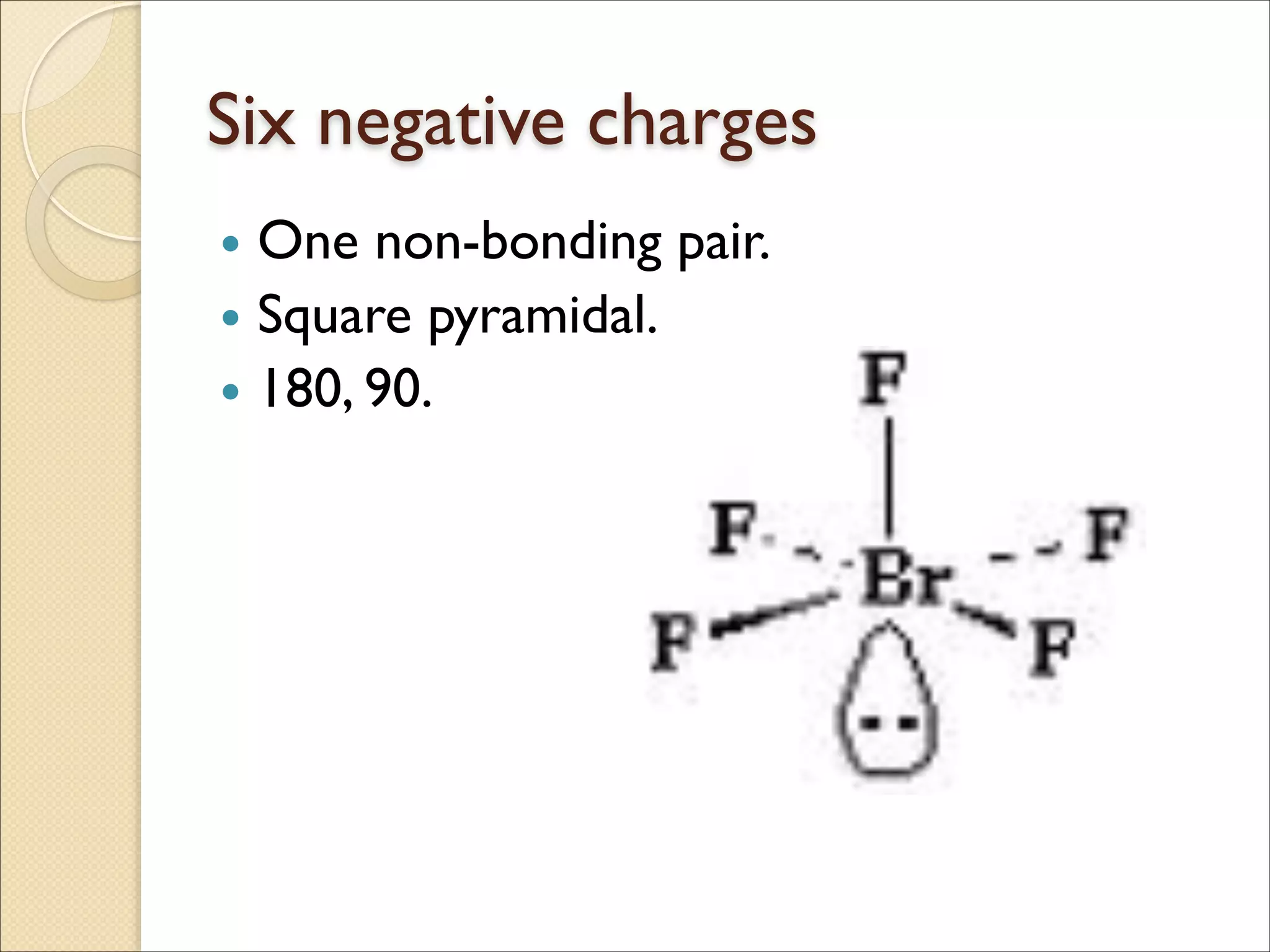
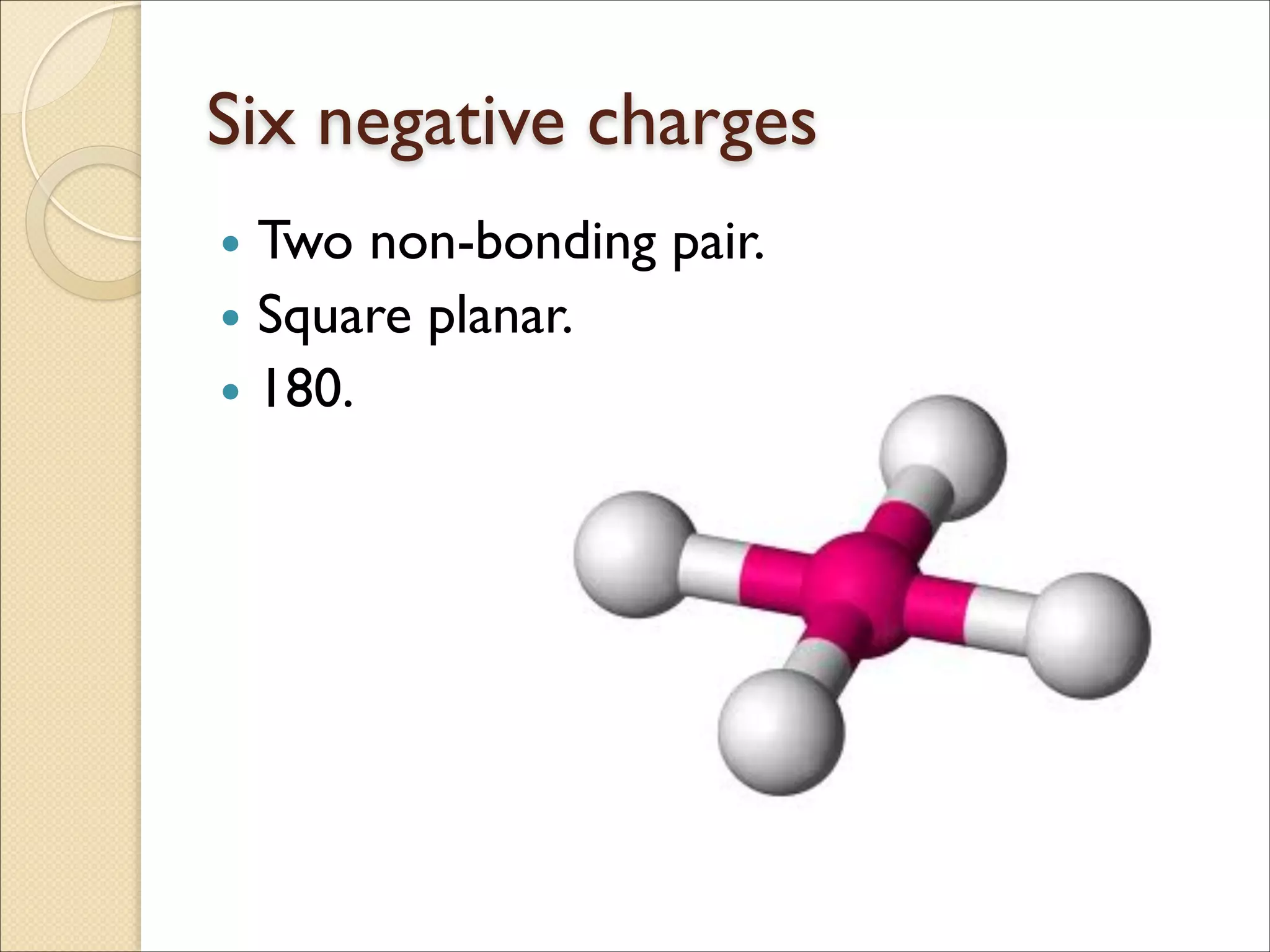
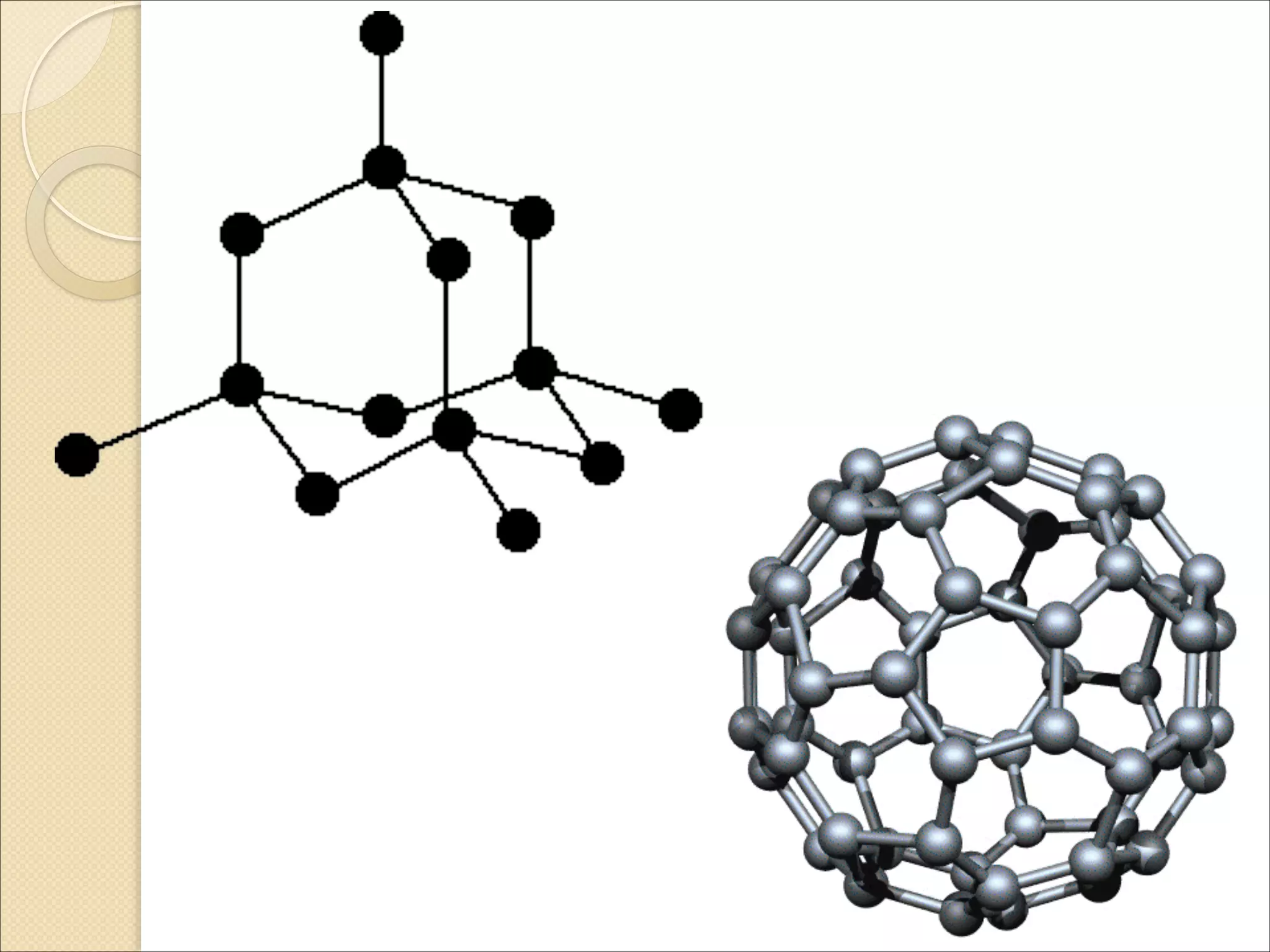
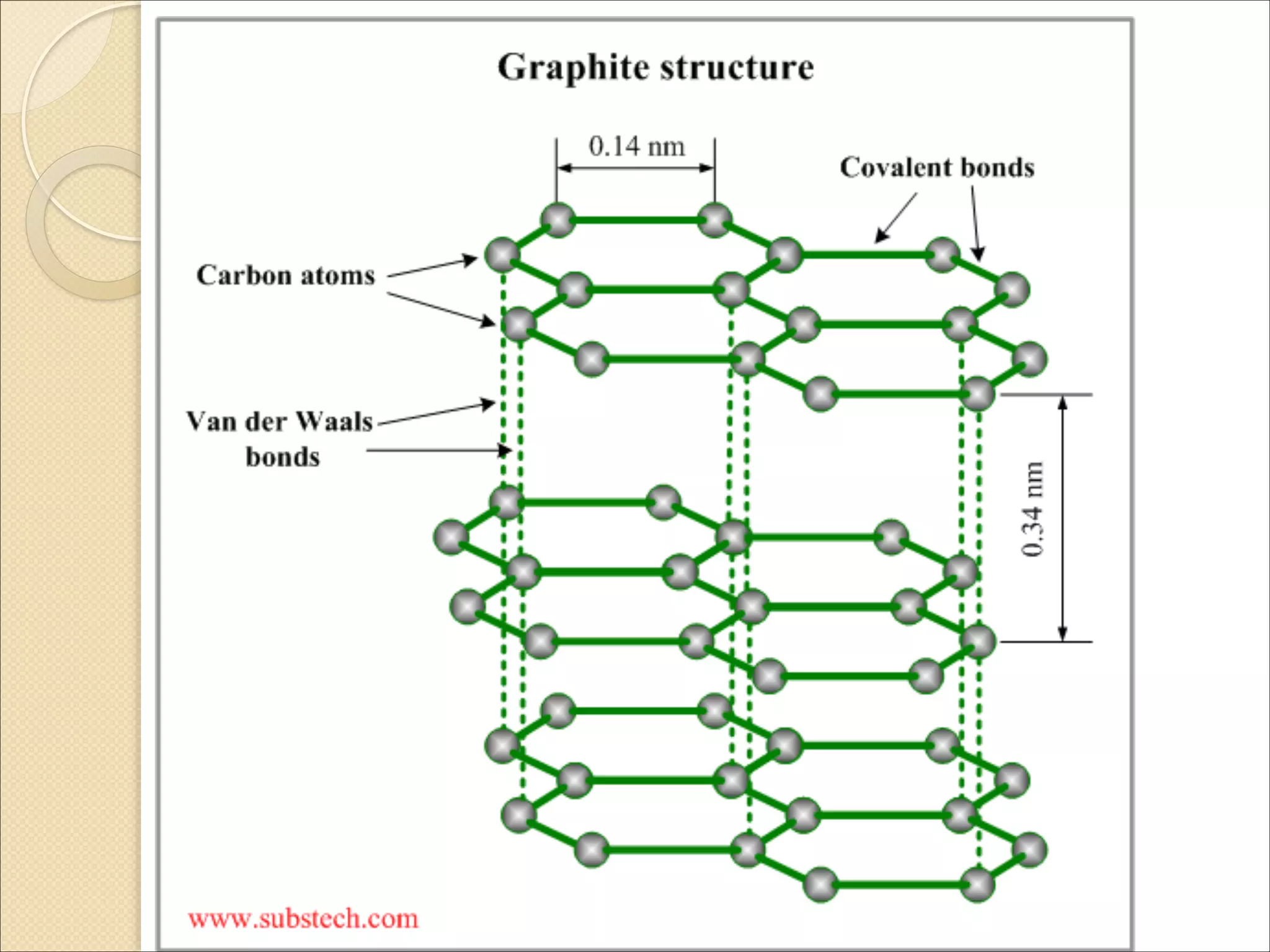
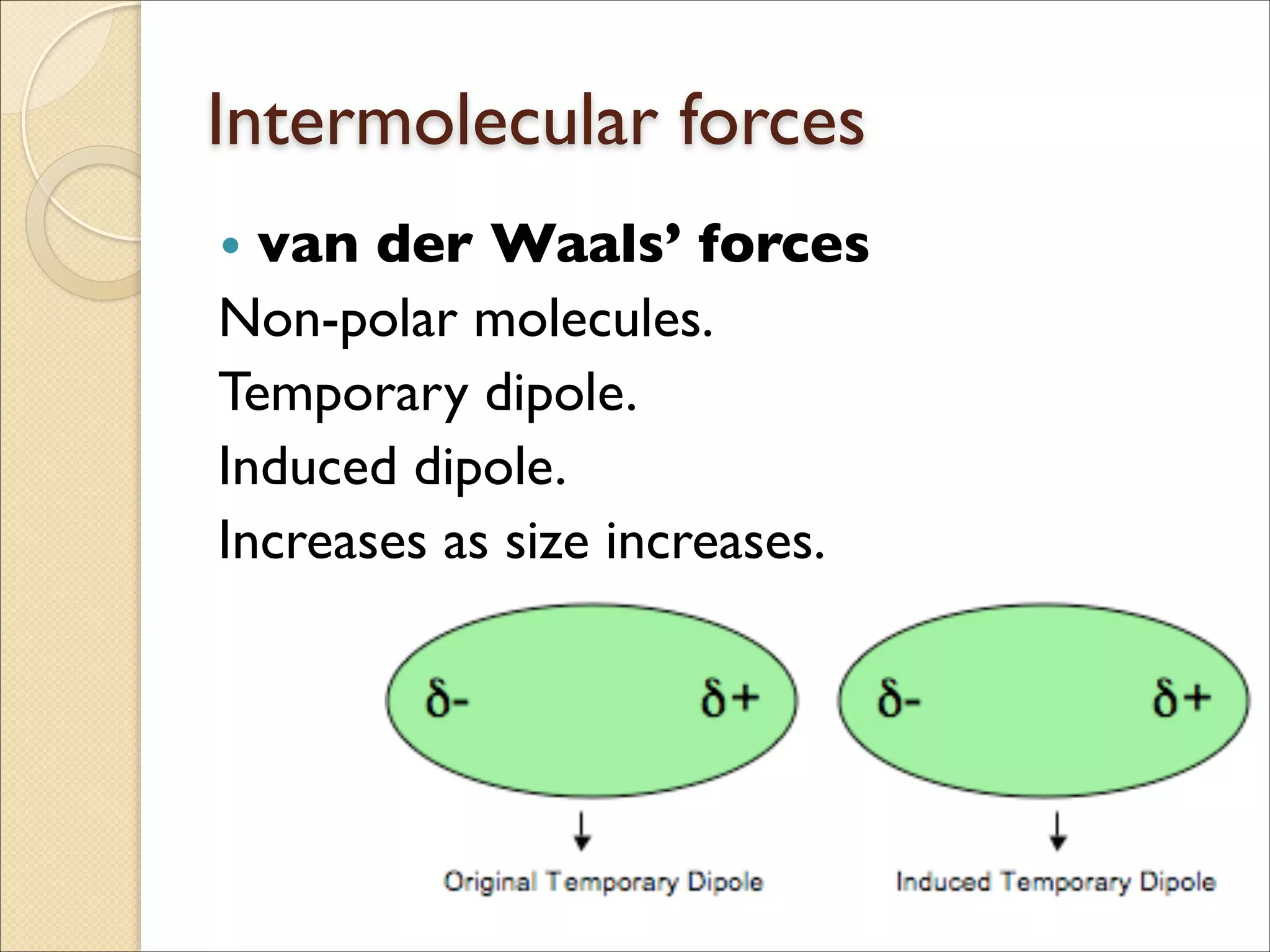
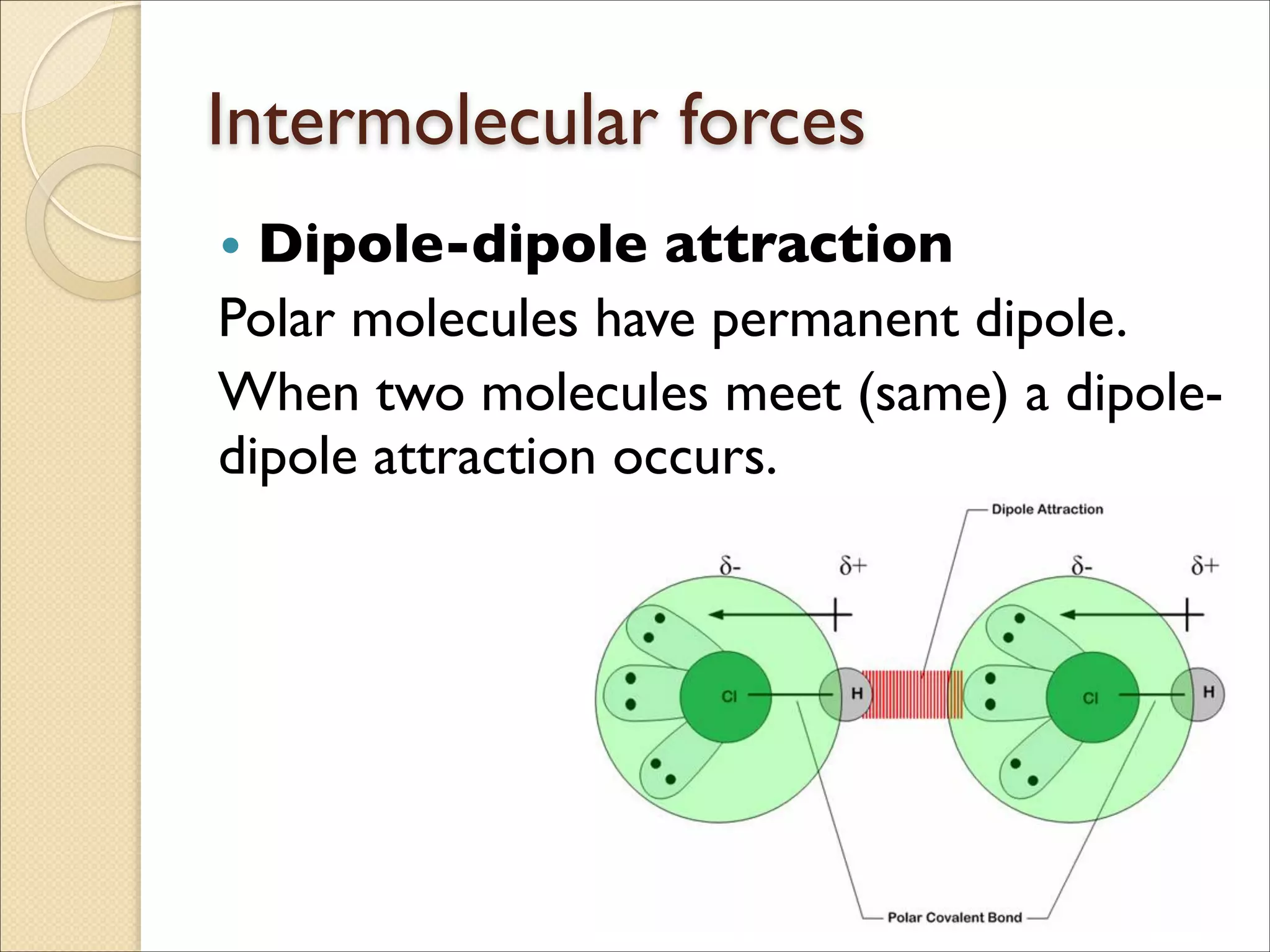
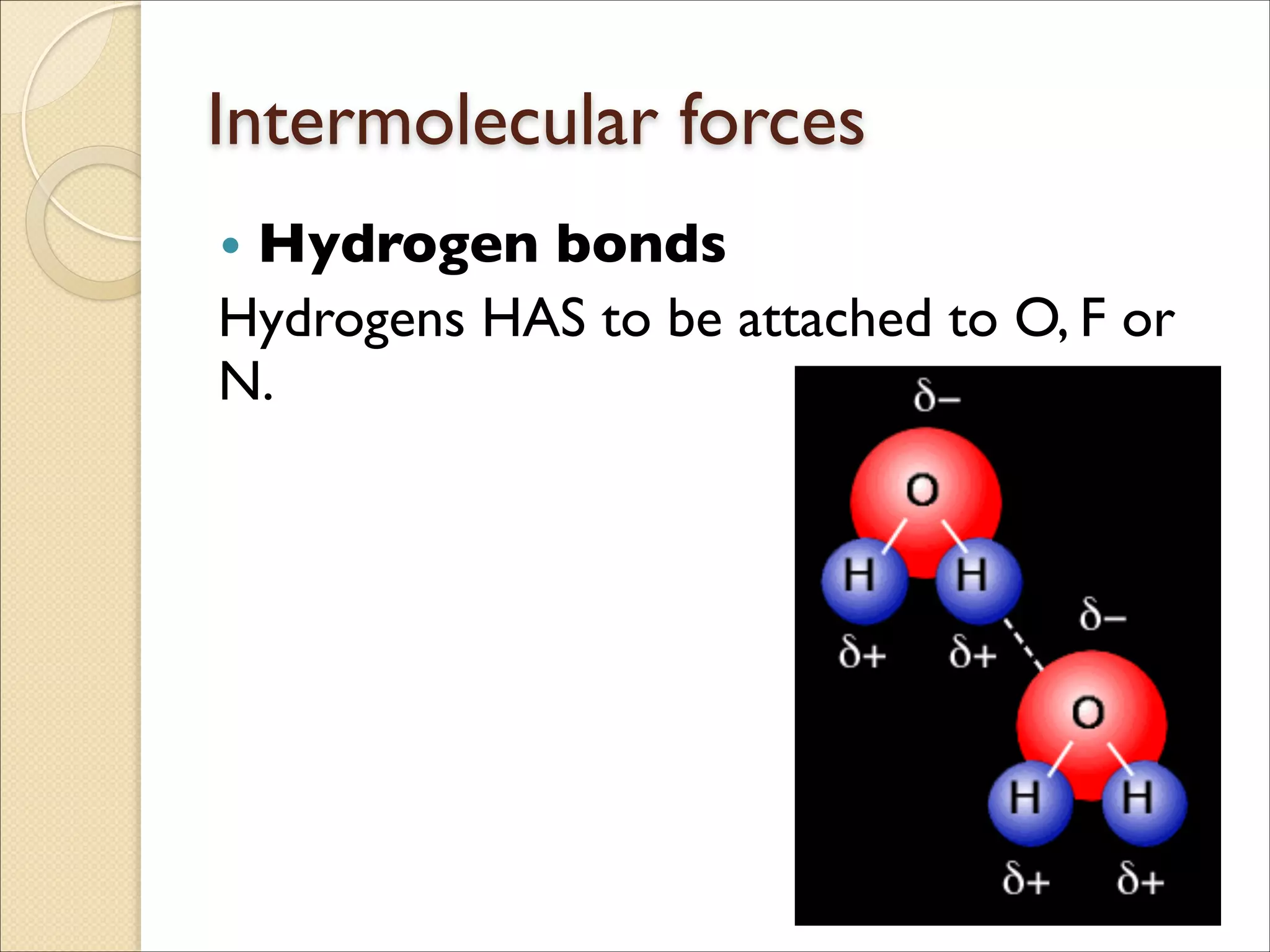
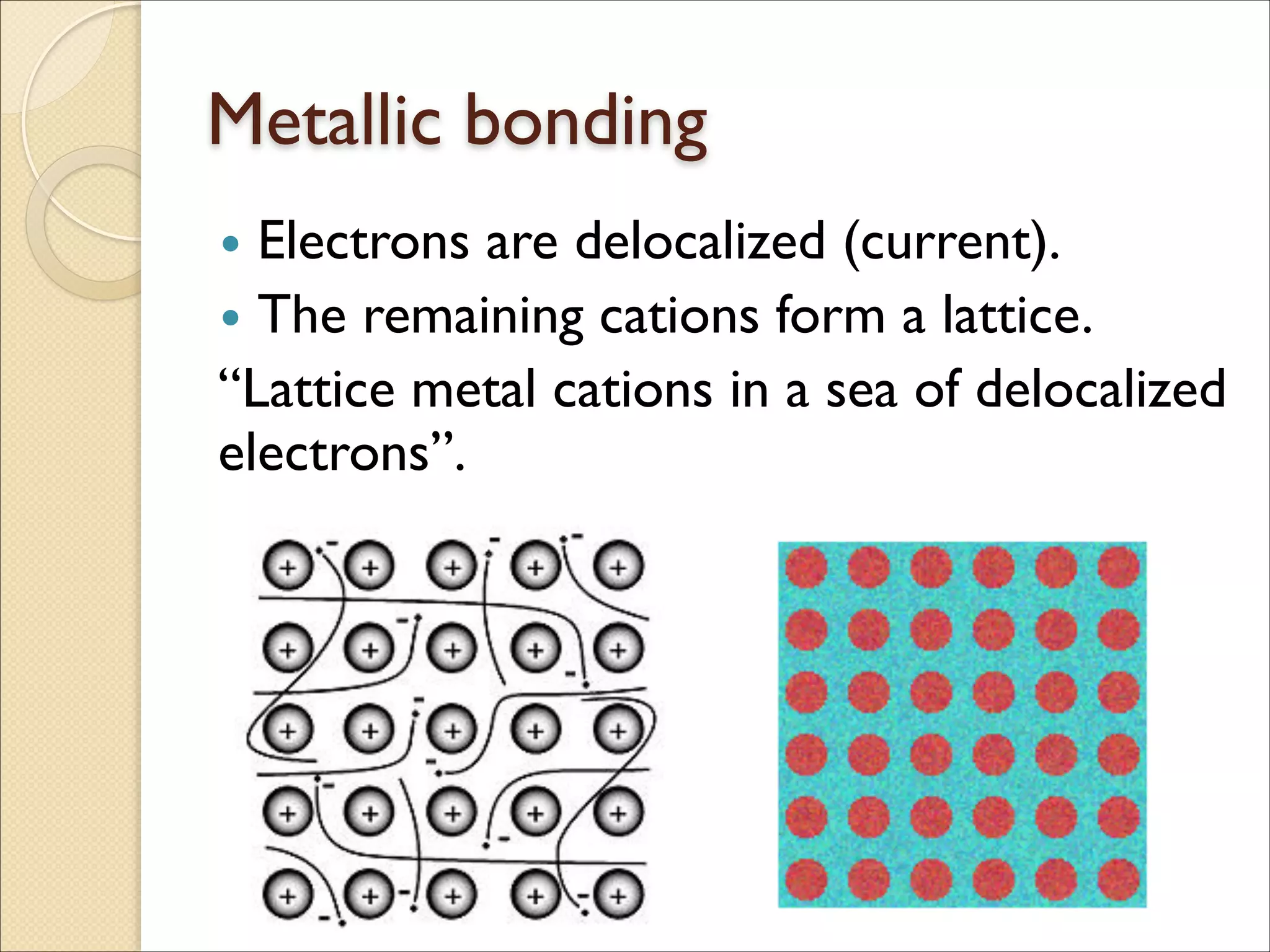
The document outlines the characteristics and formation of ionic bonds, emphasizing the electrostatic attraction between oppositely charged ions and factors such as electronegativity and periodic table positions. It also discusses covalent bonds, molecular geometry through VSEPR theory, and intermolecular forces like hydrogen bonds. Additionally, it addresses properties like solubility, melting points, and electrical conductivity of compounds.