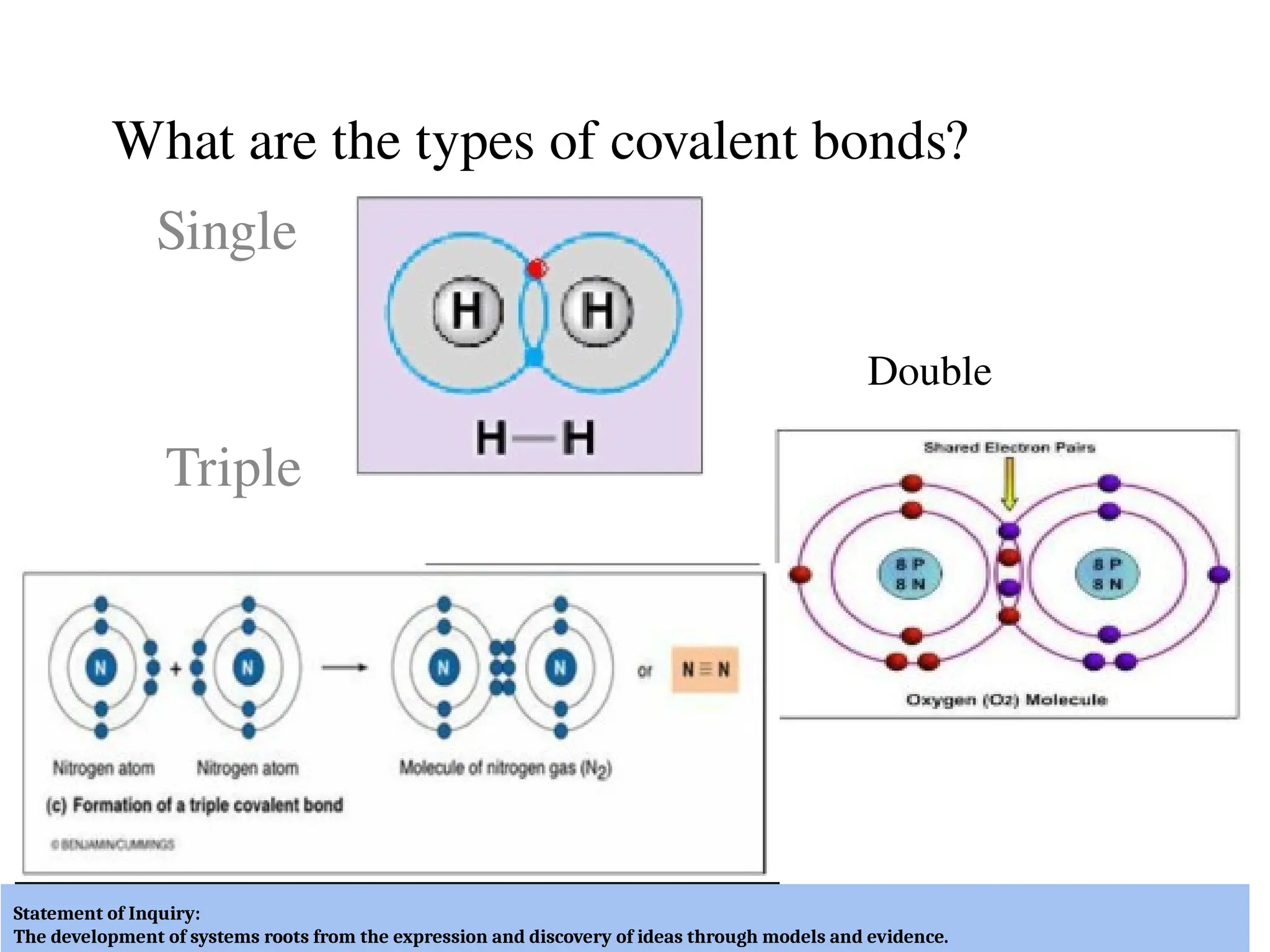
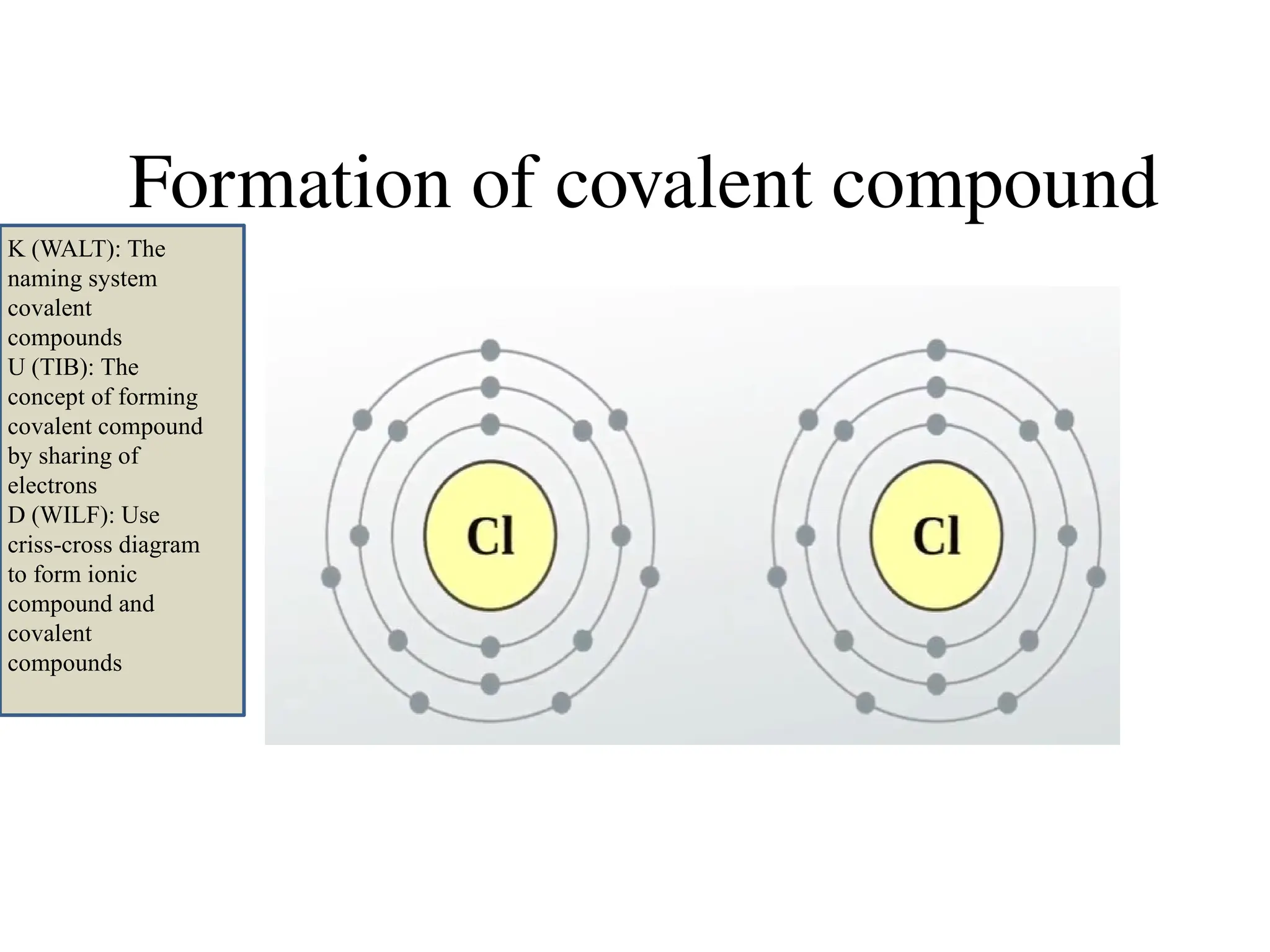
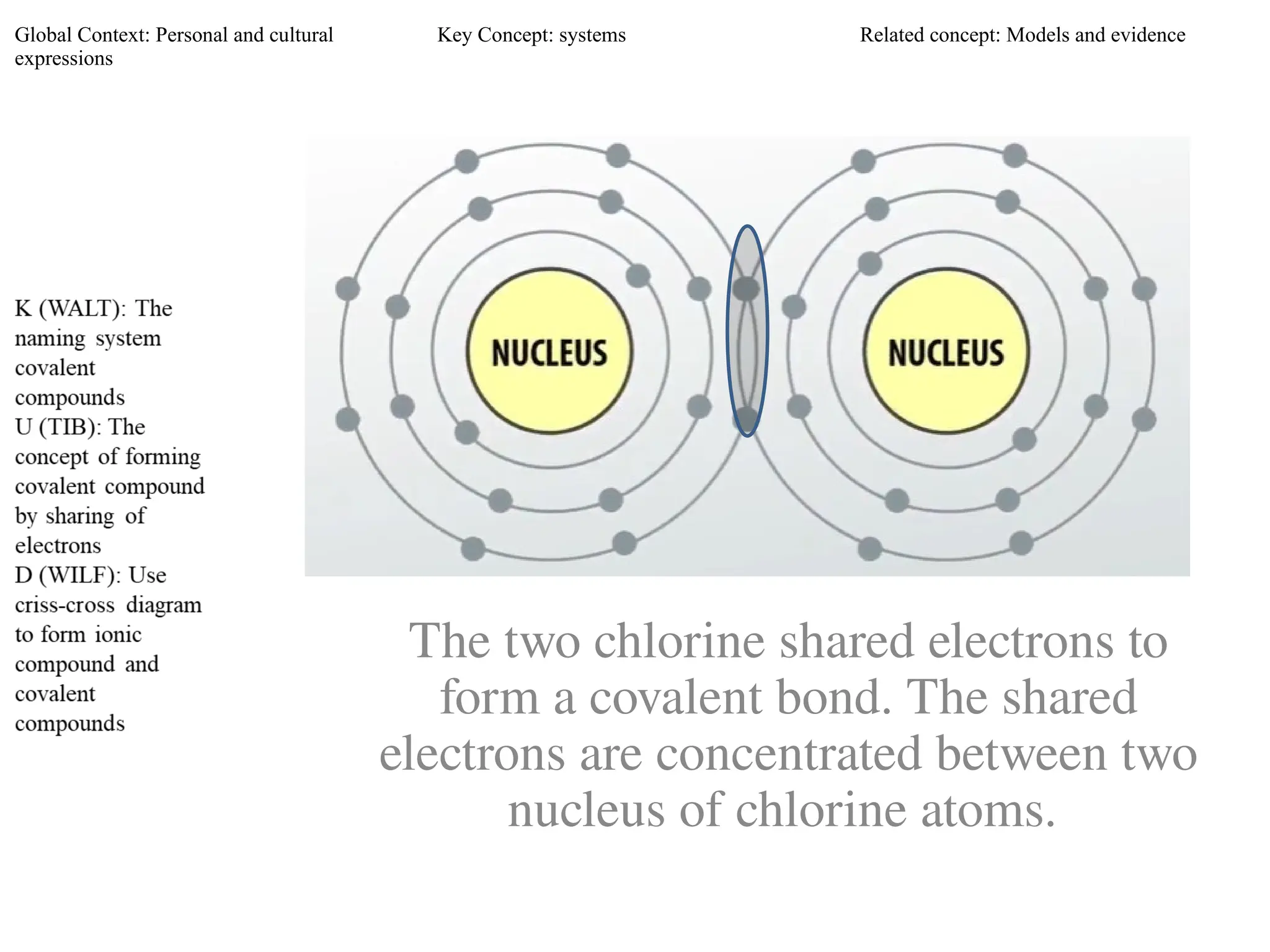
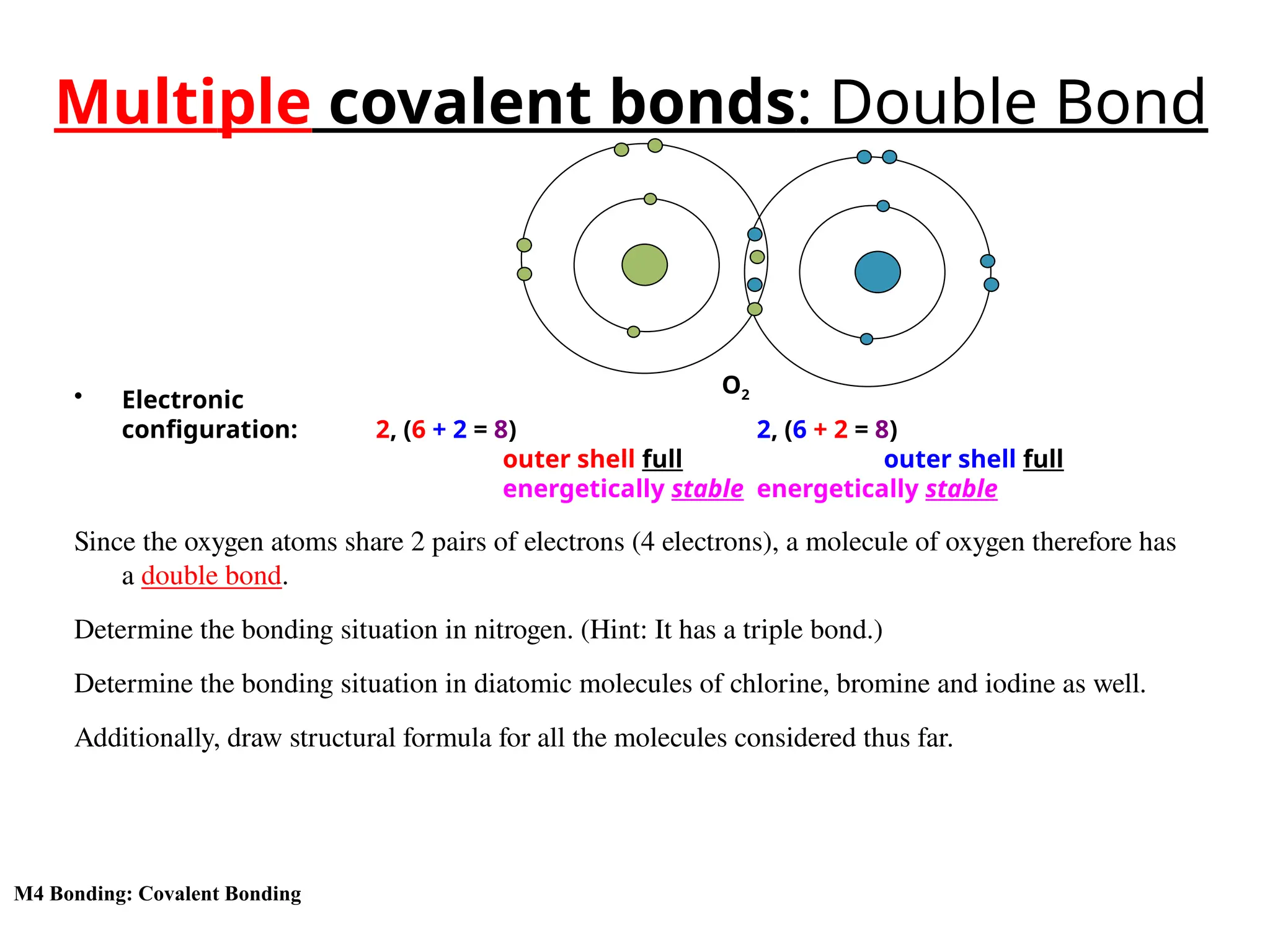
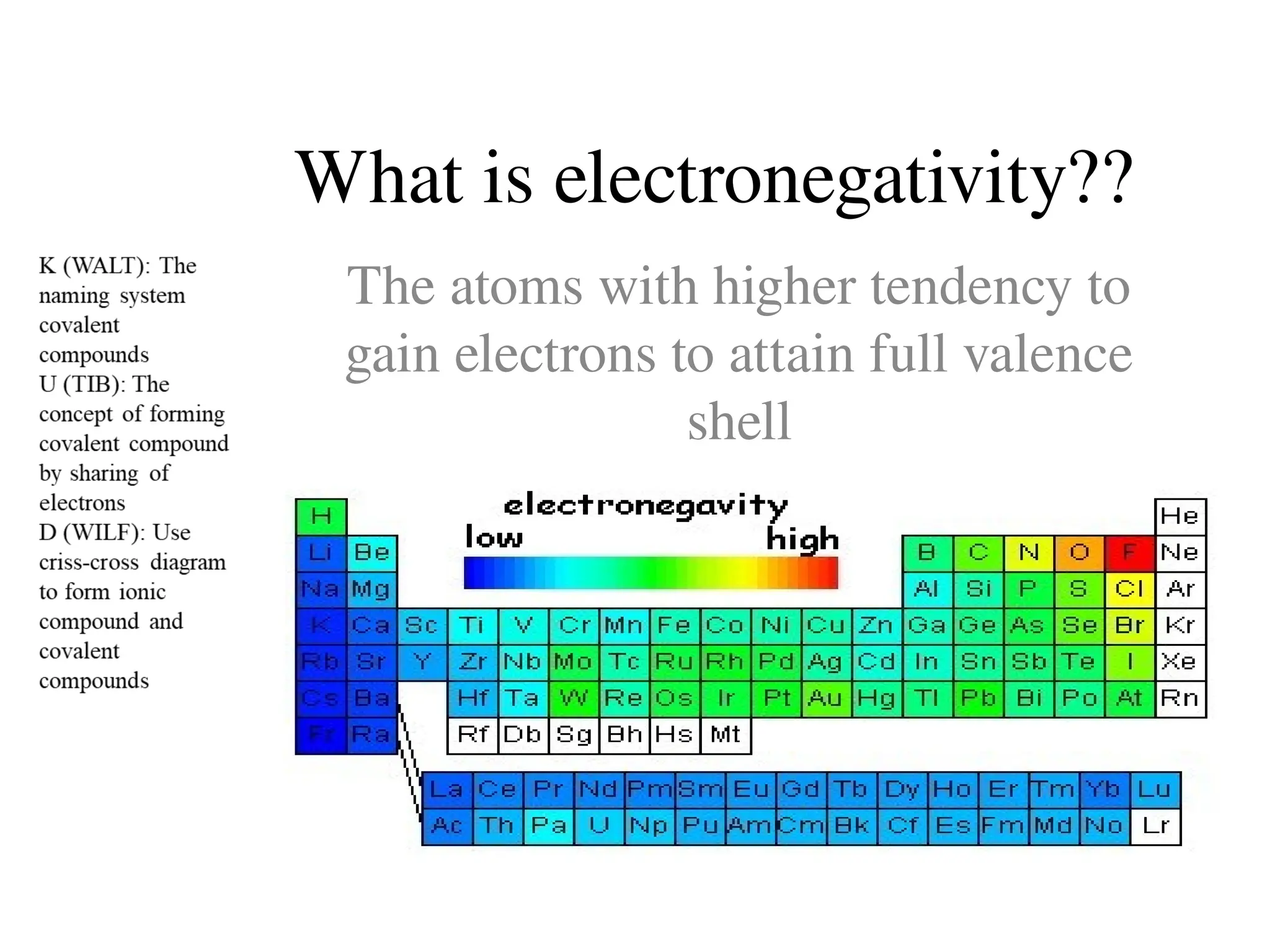
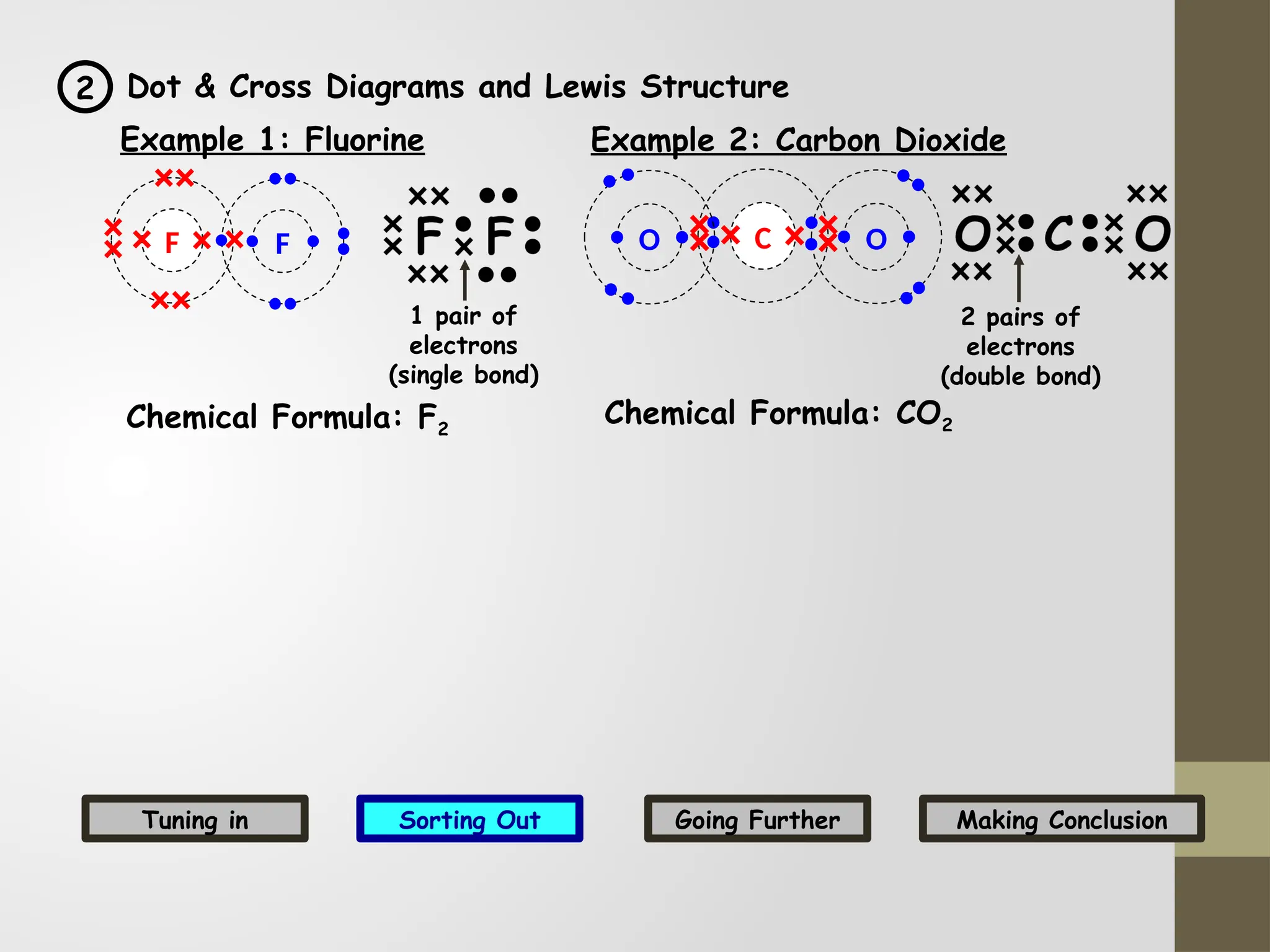
The document discusses the formation, properties, and naming of covalent compounds and bonds, emphasizing the sharing of electrons among non-metallic elements to achieve stable electronic configurations. It outlines various types of covalent bonds, including single, double, and triple bonds, and describes the characteristics of covalent compounds such as low melting/boiling points and poor conductivity. Additionally, it introduces concepts like electronegativity and polar/non-polar bonds, alongside practical examples and diagrams for better understanding.