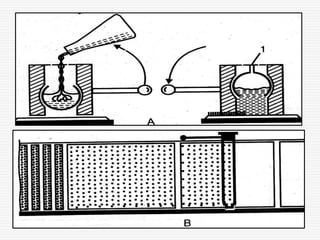
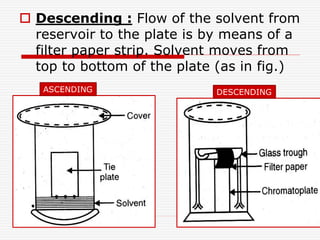
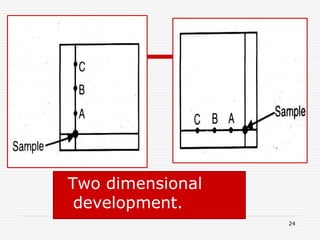
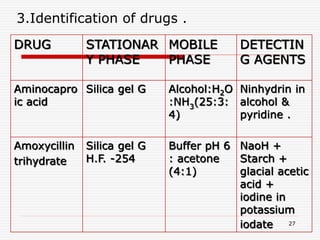
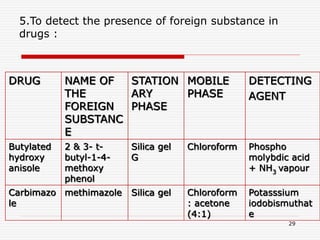
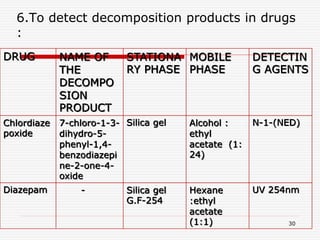
The document presents a detailed overview of thin layer chromatography (TLC), outlining its principles, procedures, and applications in the analysis of various compounds. TLC is characterized by the separation of compounds based on their affinity to stationary and mobile phases, using adsorbents on a glass plate. Applications include the separation and identification of drugs, carbohydrates, and other organic substances, with various detection methods described.