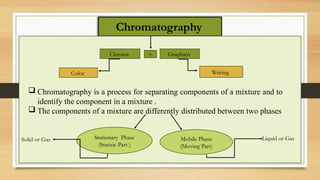
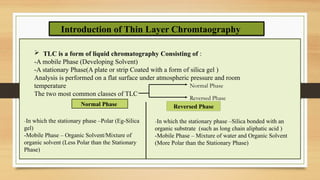
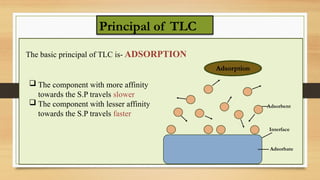
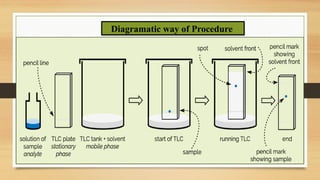
This presentation provides an in-depth overview of Thin Layer Chromatography (TLC), a widely used analytical technique in chemistry and pharmaceutical sciences for separating and identifying compounds. It explains the principle, procedure, and applications of TLC, along with its advantages and limitations.
Topics Included-
Principle and working mechanism of TLC
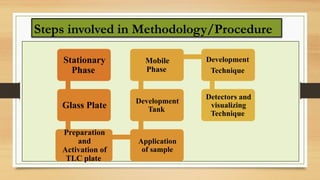
Components of TLC: stationary phase, mobile phase, and sample
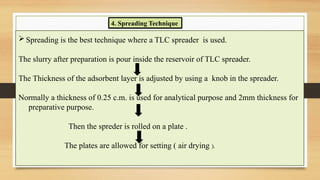
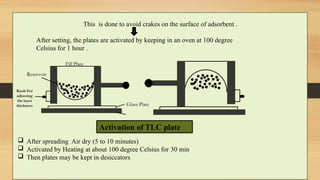
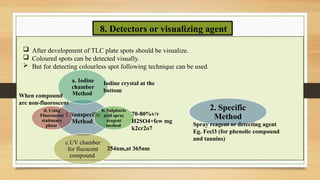
Step-by-step experimental procedure
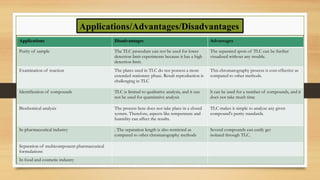
Applications in pharmaceutical analysis and quality control
Advantages, limitations, and comparison with other chromatographic techniques