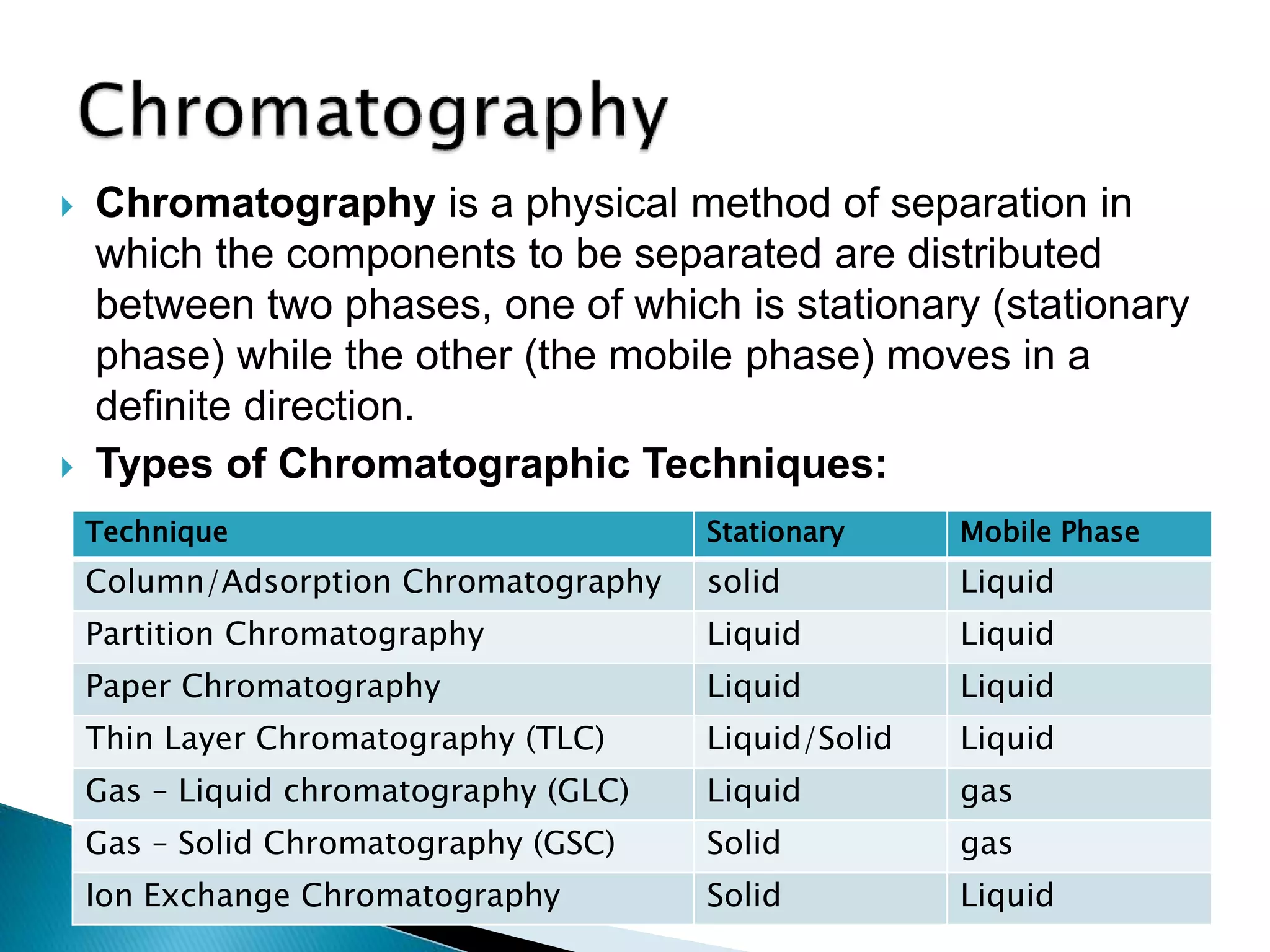
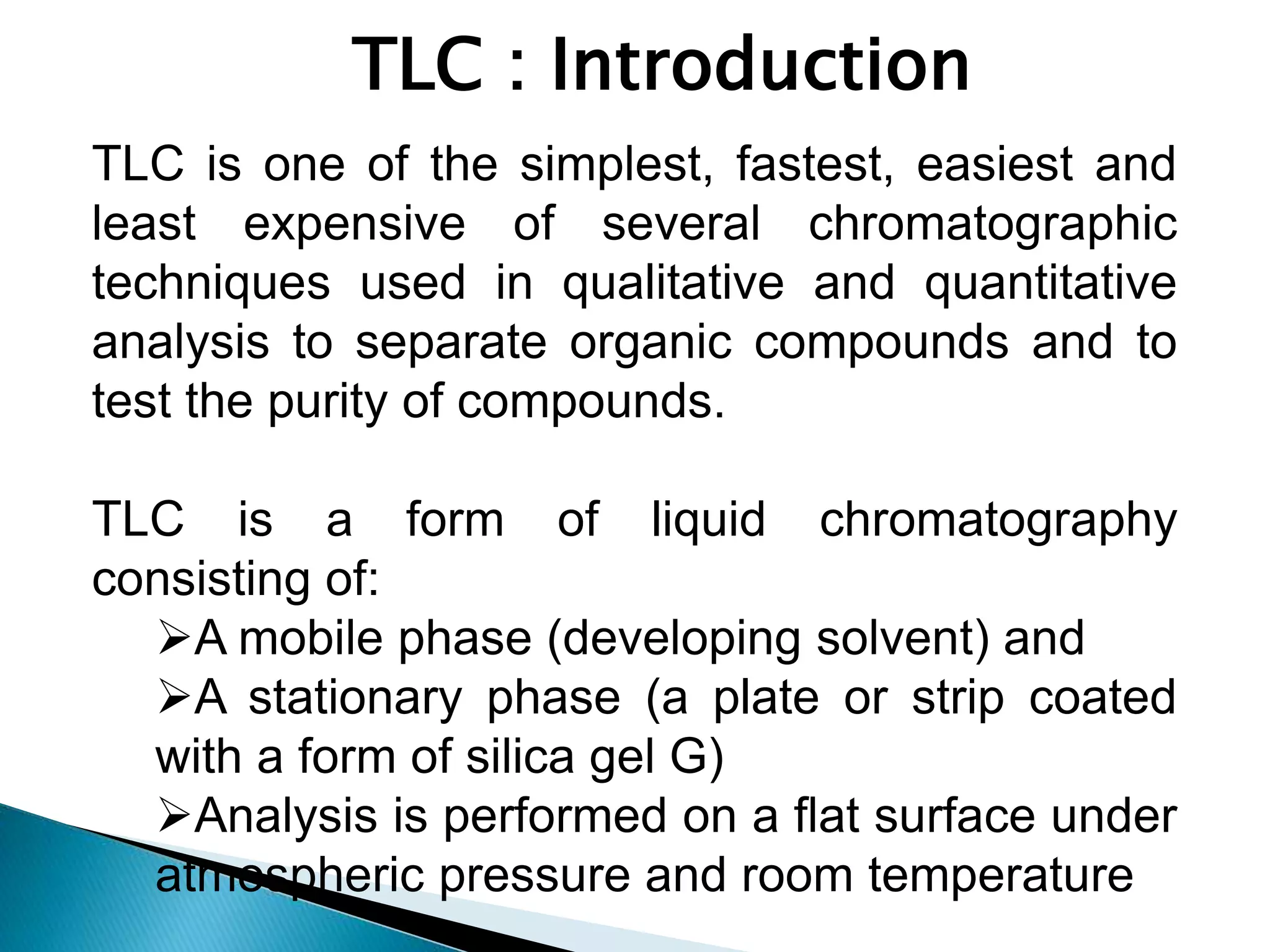
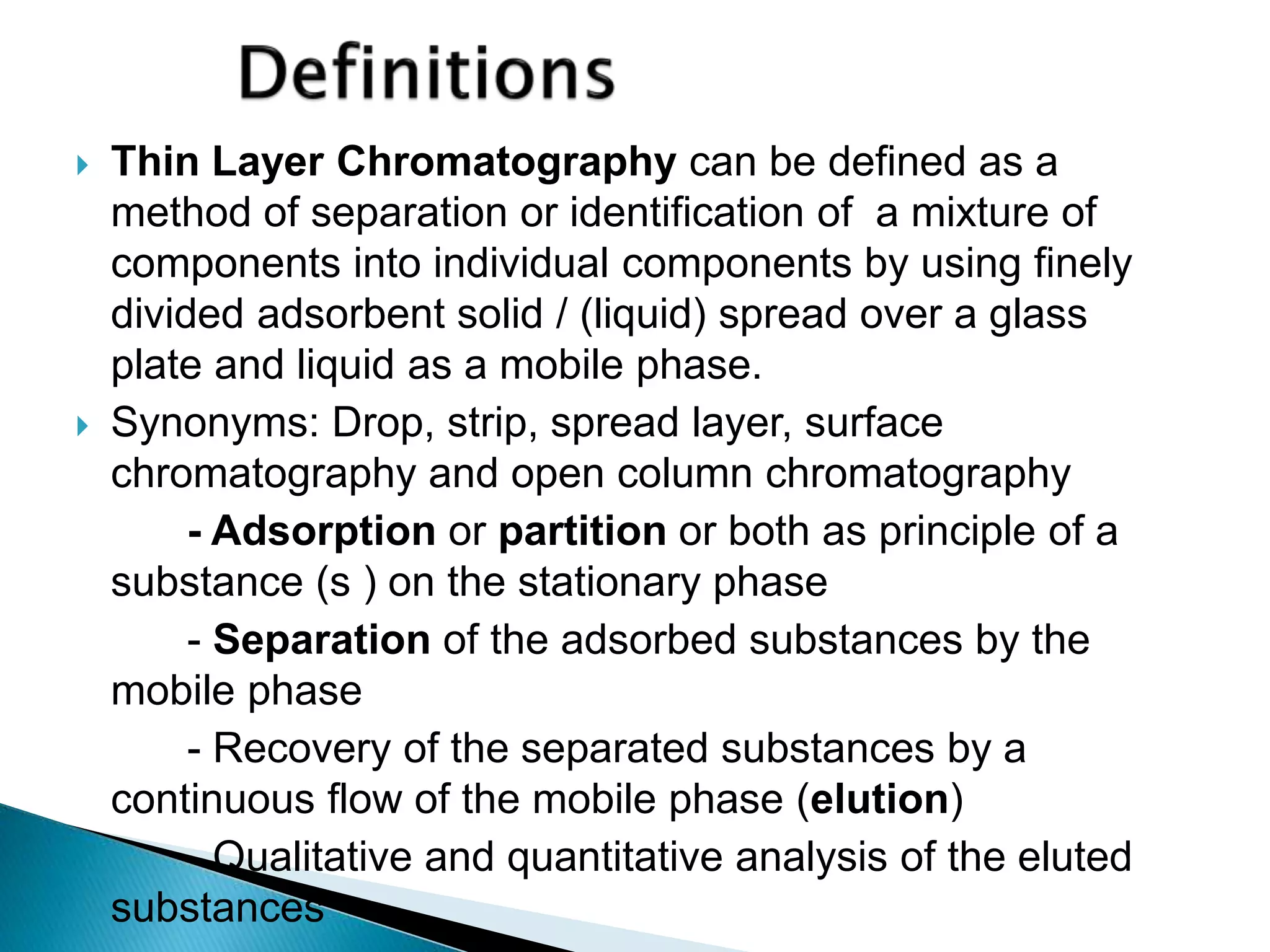
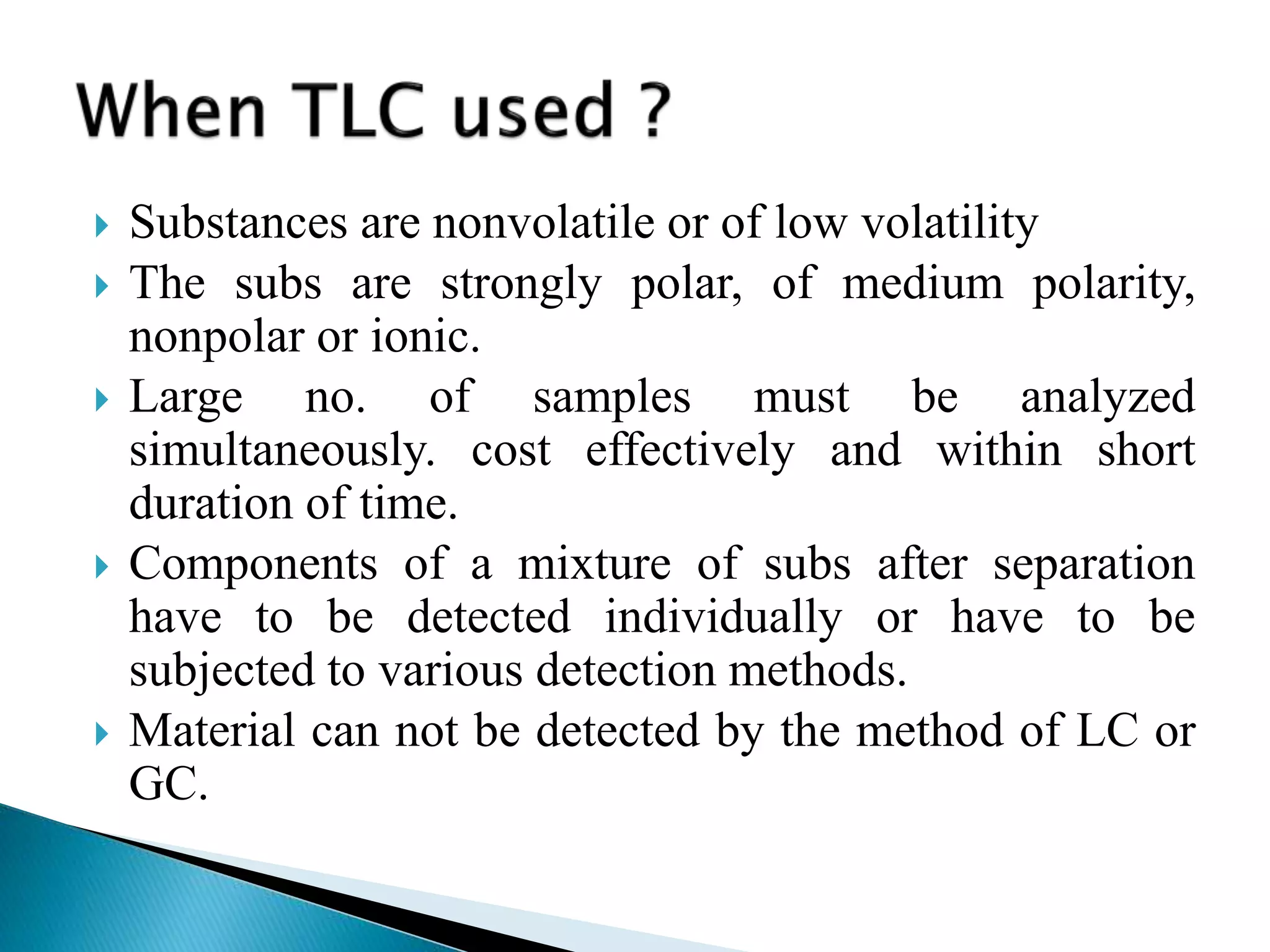
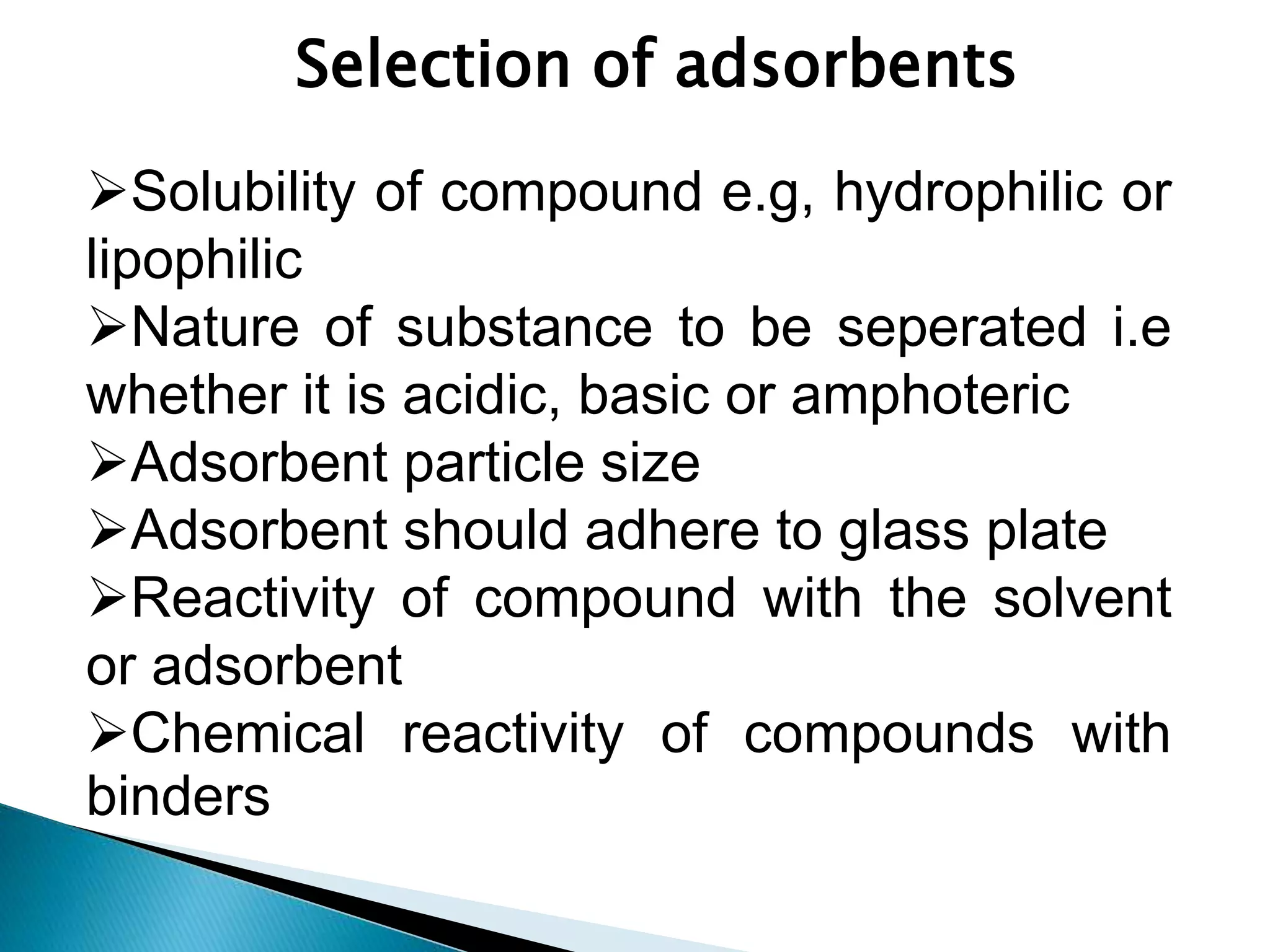
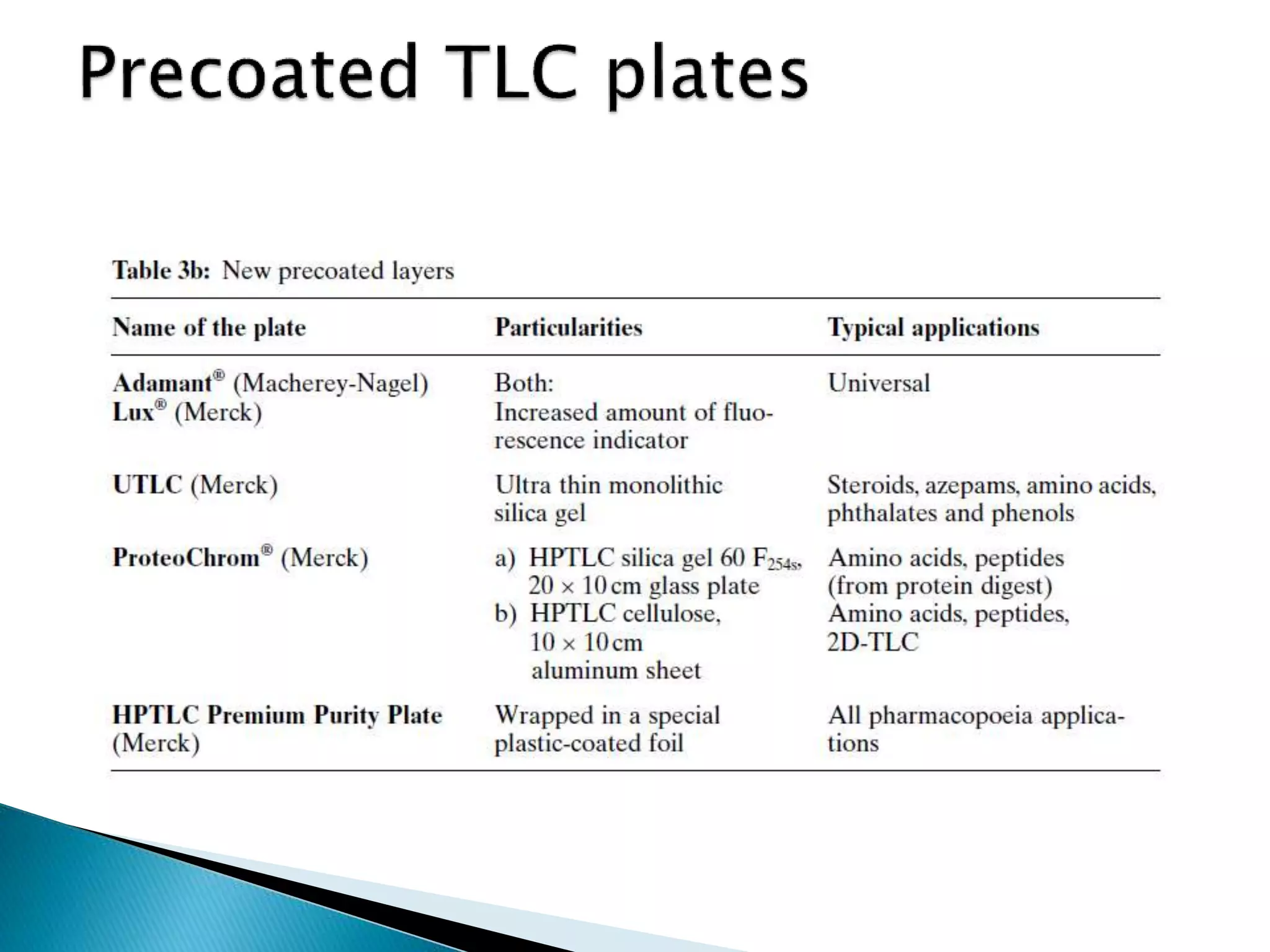
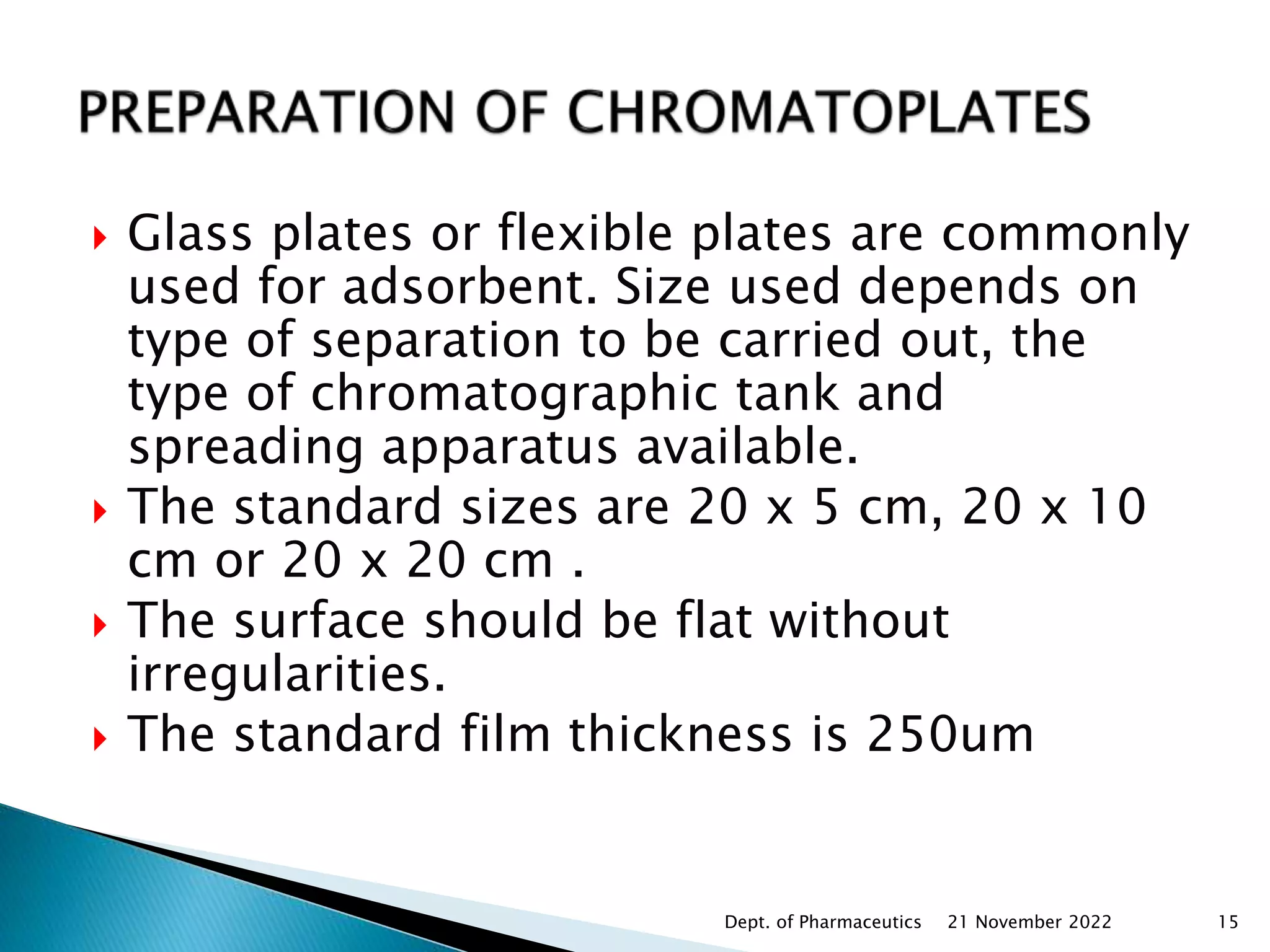
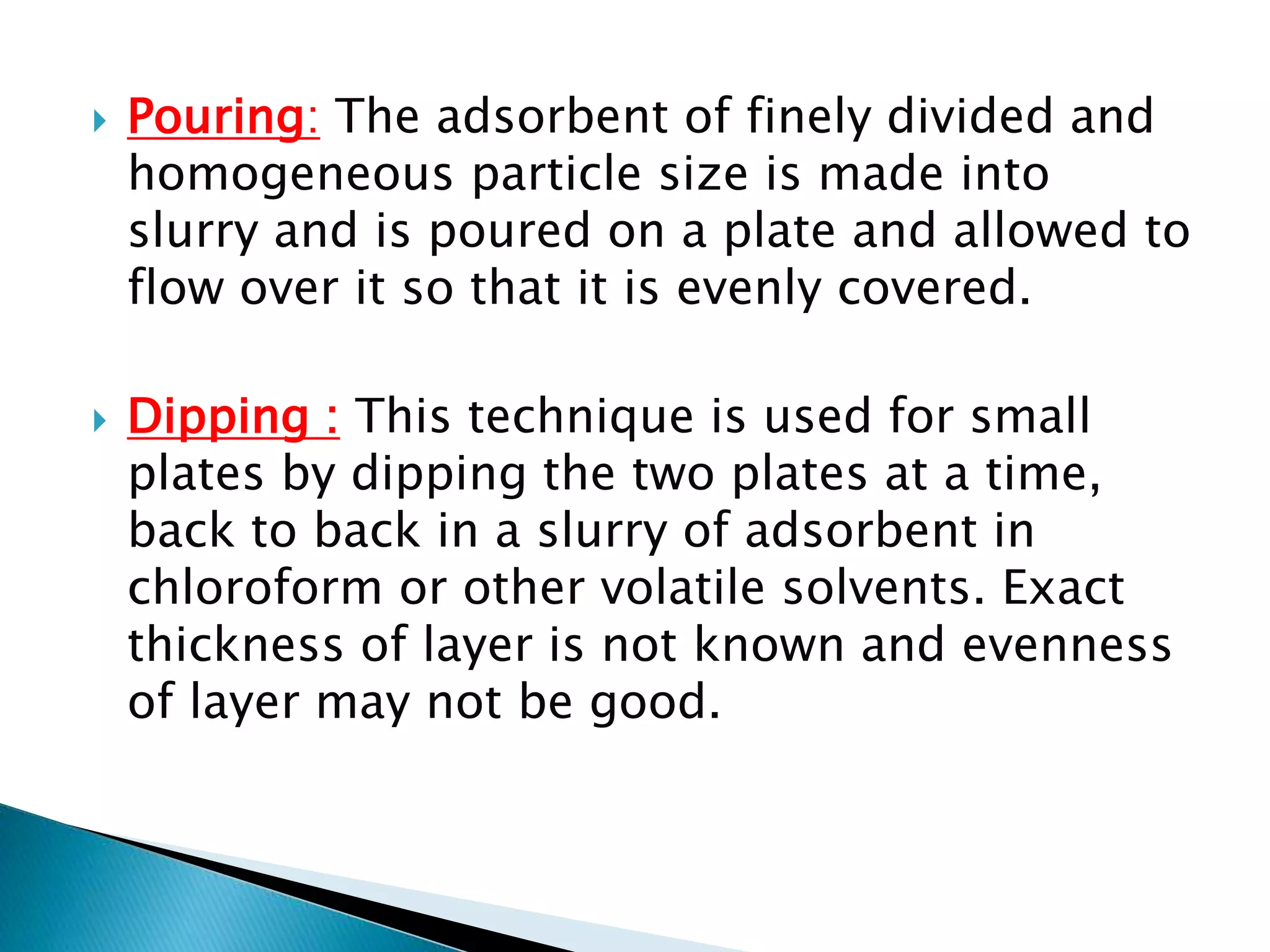
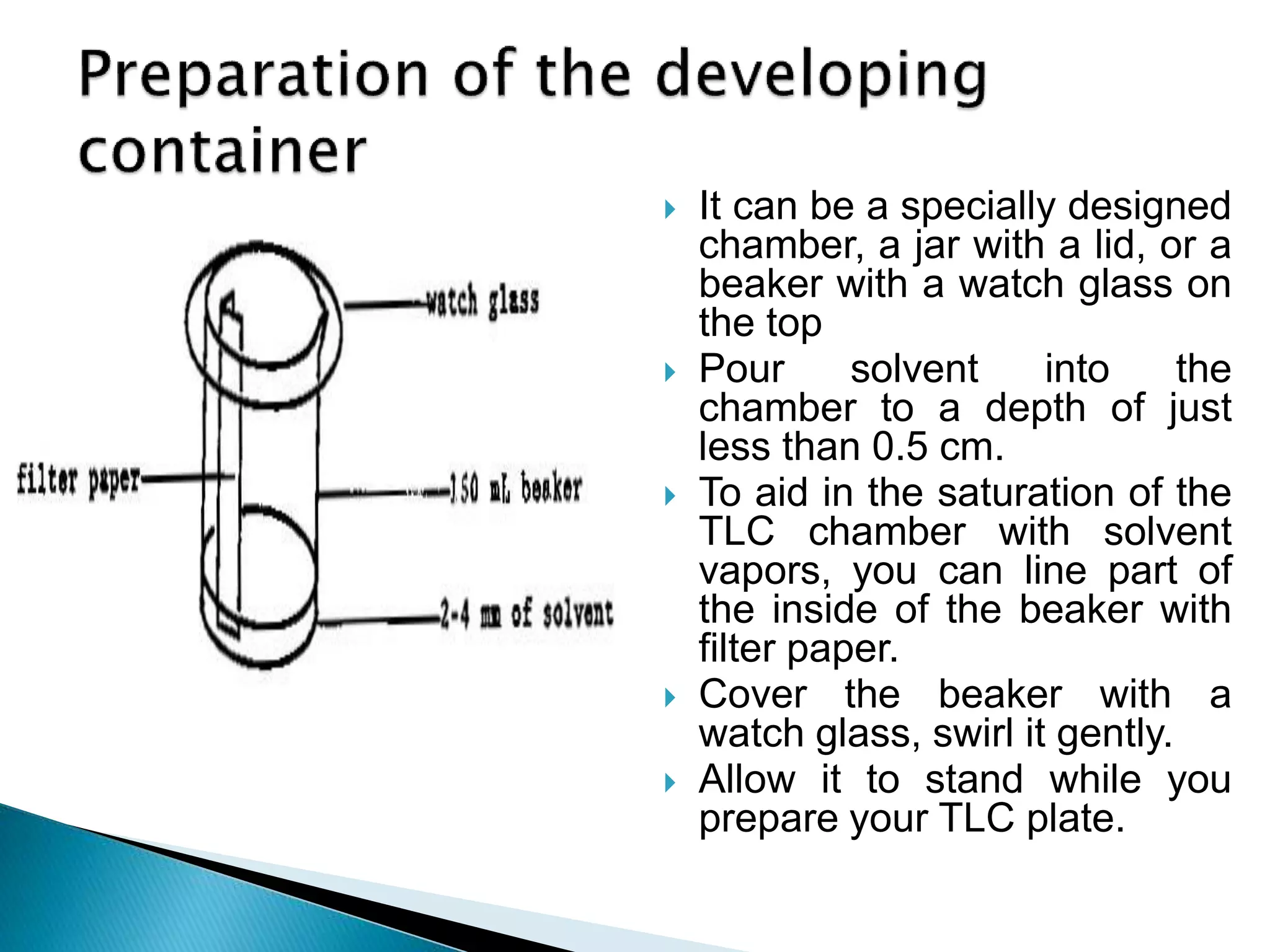
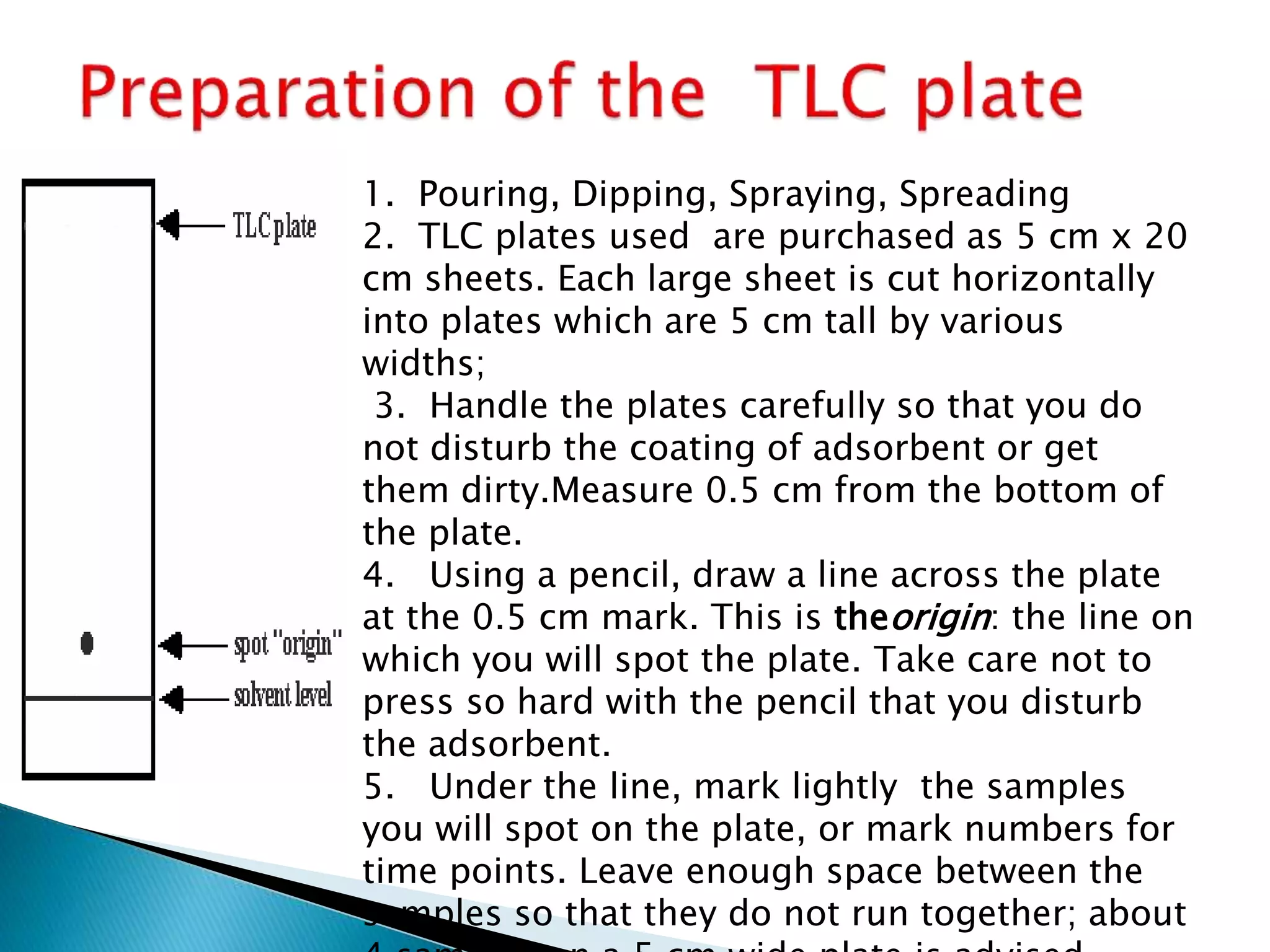
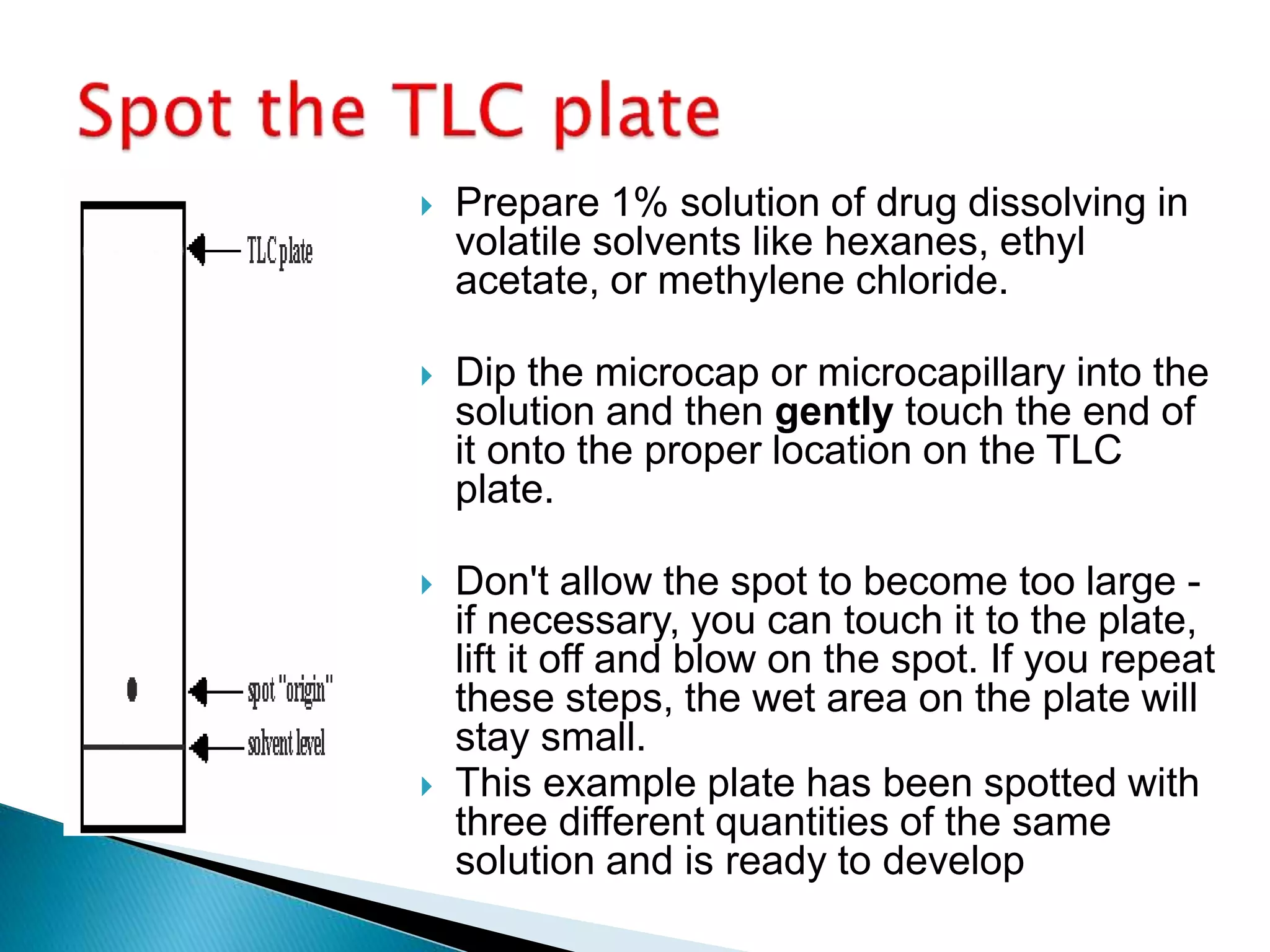
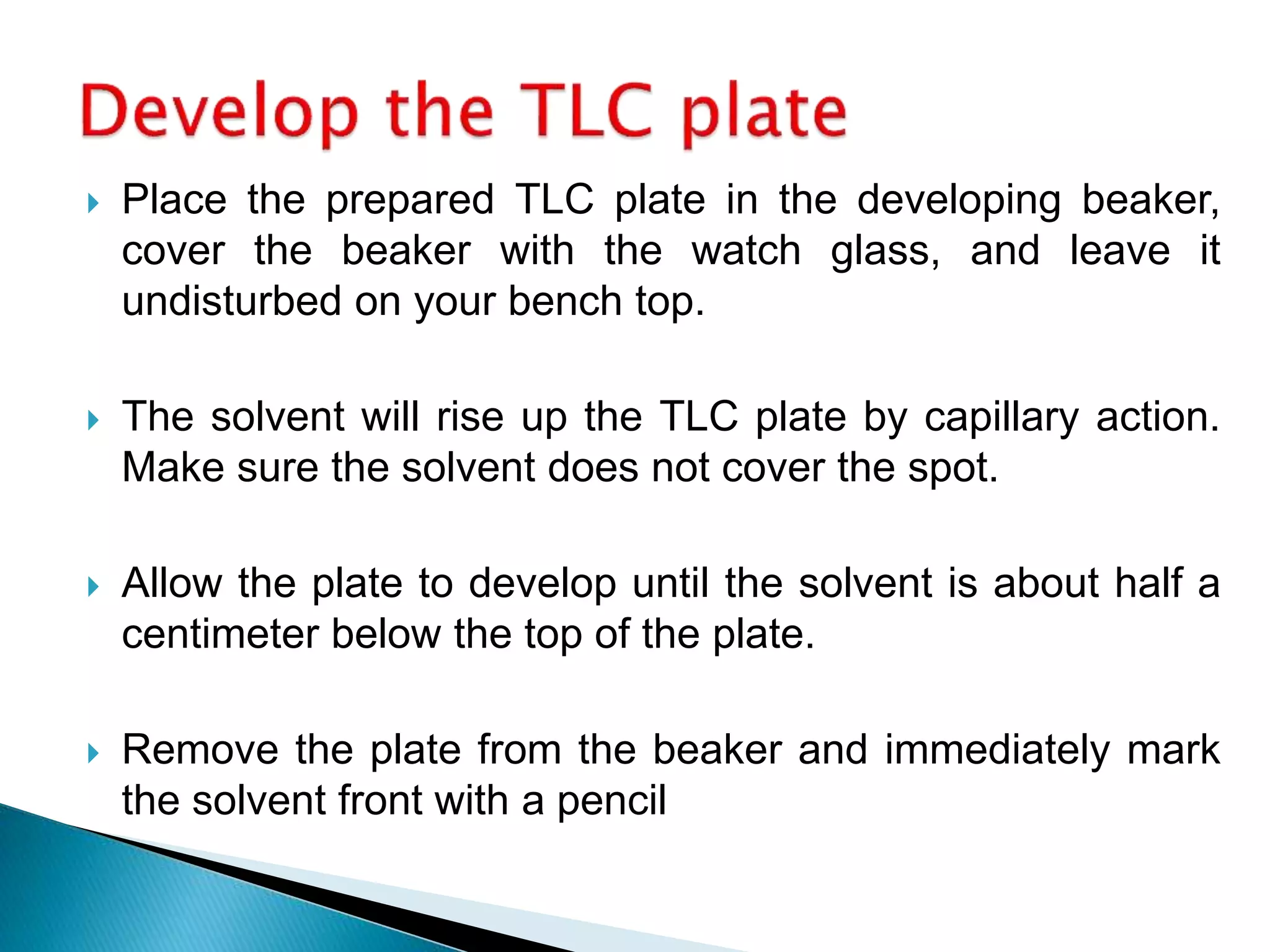
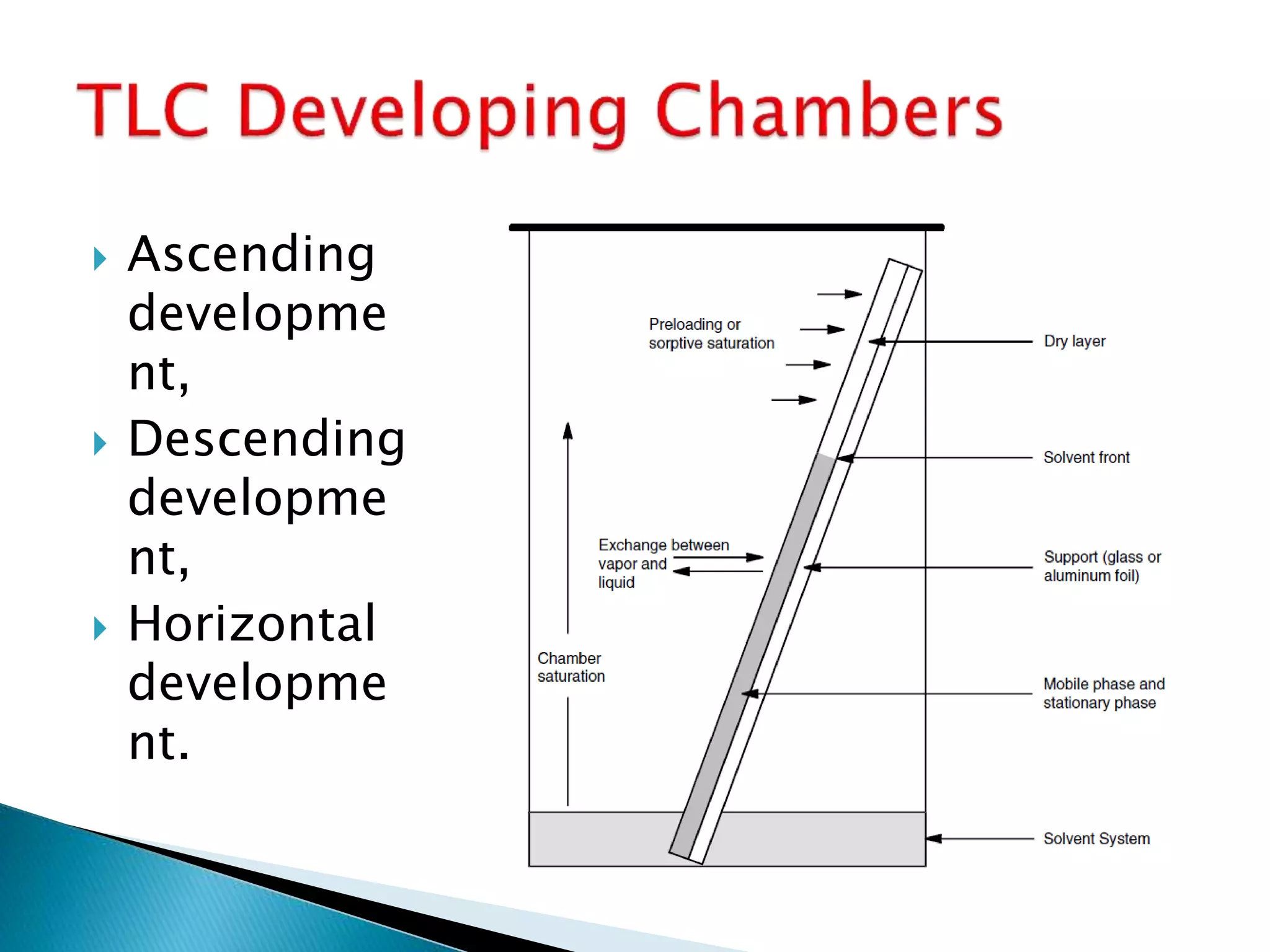
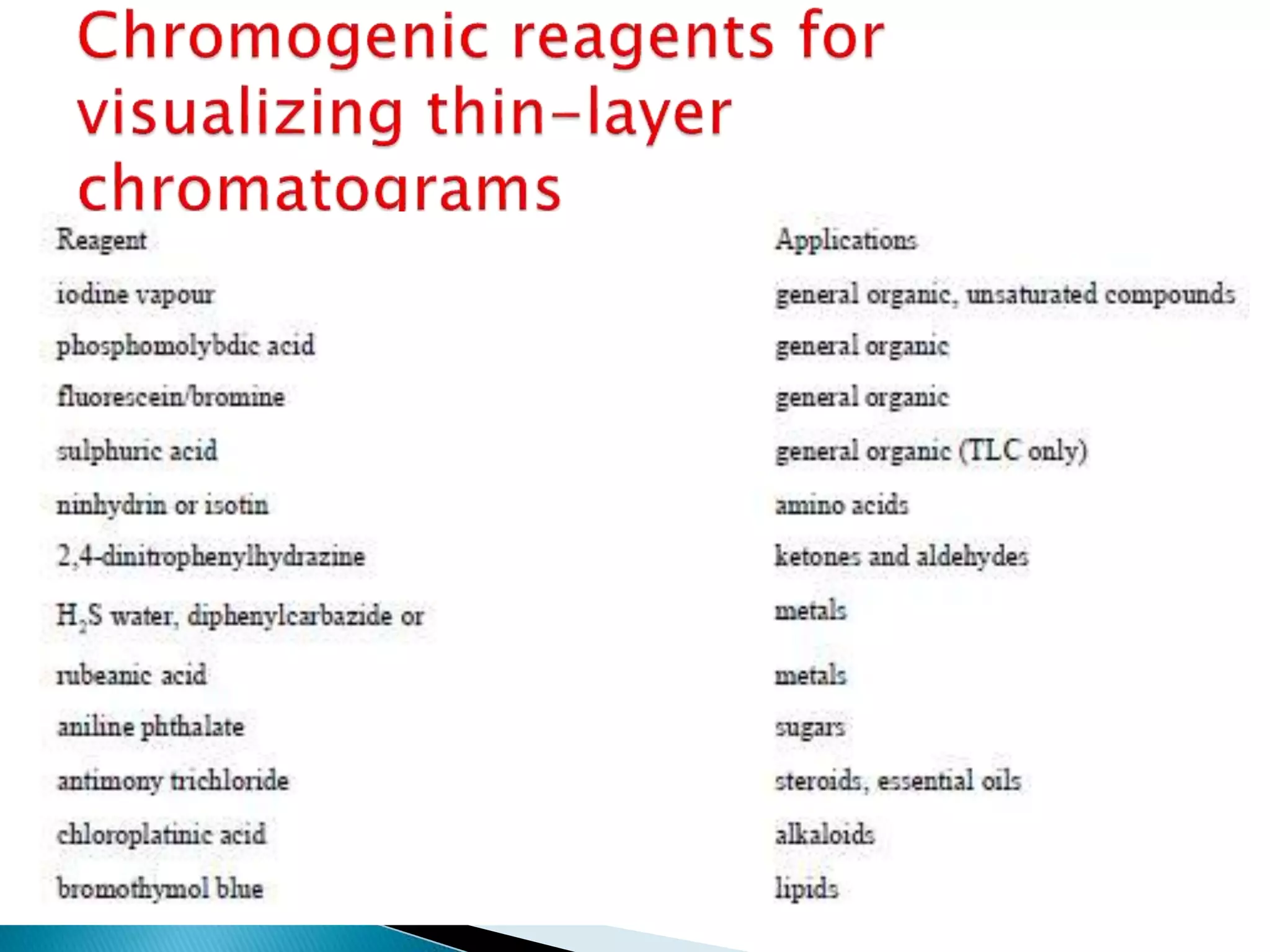
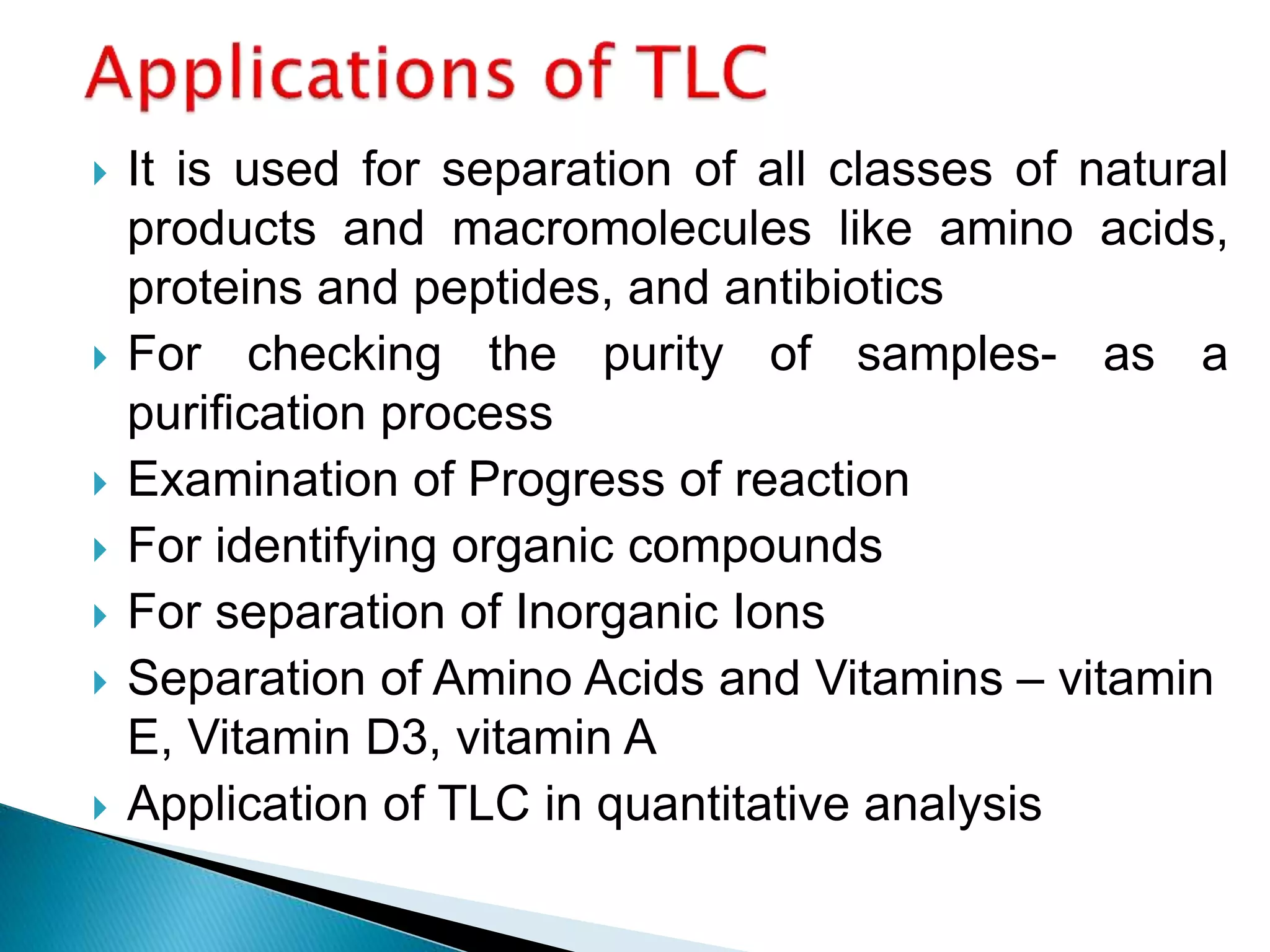
Chromatography is a technique used to separate mixtures by distributing components between a stationary and mobile phase. Thin layer chromatography (TLC) is a type of liquid chromatography that uses a thin layer of adsorbent material, like silica gel, coated on a flat surface as the stationary phase. A liquid mobile phase is then used to separate the components of a mixture applied to the plate as they migrate at different rates. Factors like the choice of stationary and mobile phases, and development conditions impact the separation achieved by TLC. It is a simple, fast, and inexpensive technique used for qualitative and quantitative analysis of mixtures.