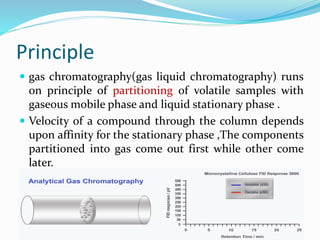
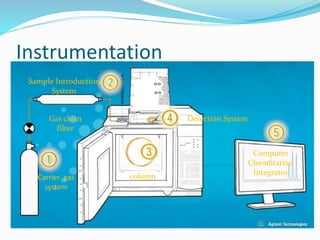
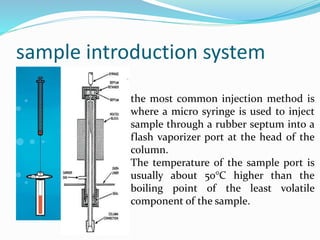
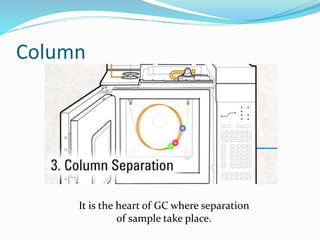
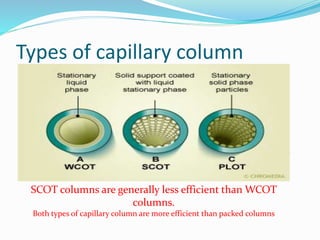
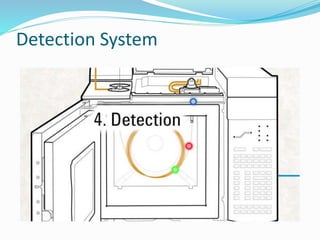
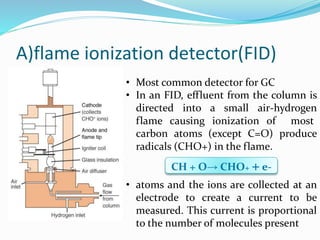
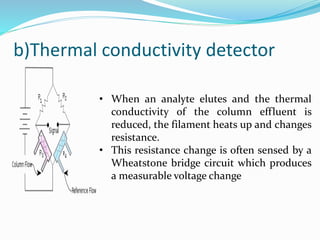
Gas chromatography is a technique used to separate and analyze compounds that can be vaporized. It works by partitioning samples between a gas mobile phase and liquid stationary phase in a column. The separation depends on how the compounds partition between the phases. Key components of a GC system include the carrier gas, sample introduction system, column for separation, and detection system such as FID or TCD. Common applications include analysis of aromatics, hydrocarbons, flavors, and other volatile organic compounds.