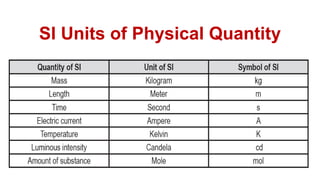
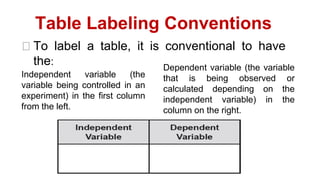
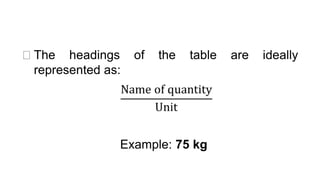
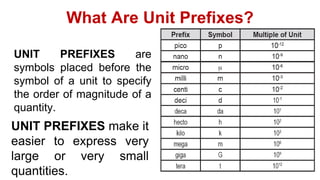
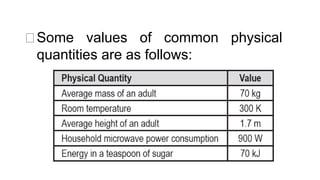
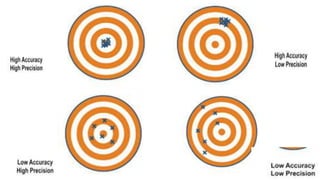
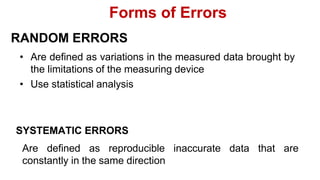
This document provides an introduction to general physics. It discusses what physics is, including concepts like mechanics, waves, electricity and magnetism. It then covers introductory physics concepts like physical quantities and units. The International System of Units (SI) is introduced as the standard system used in physics. Common units and prefixes are described. The document also discusses estimation, accuracy, precision, types of errors, and causes of error in physics experiments.