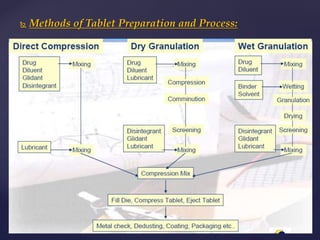
The document discusses the tablet dosage form, including its definition, advantages, disadvantages, general properties, types, ingredients, preparation methods, and evaluation processes. It highlights the benefits of tablets for precision dosing and stability, alongside challenges like difficulty in swallowing and formulation issues for certain drugs. Additionally, it details coating techniques, defects in tablets, and methods to evaluate their quality and performance.