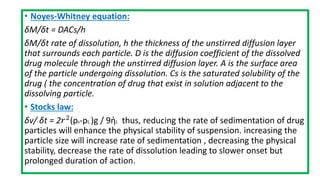
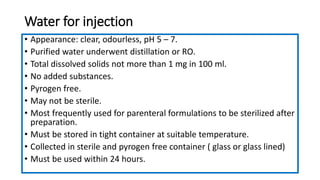
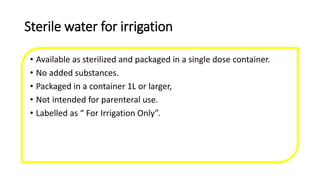
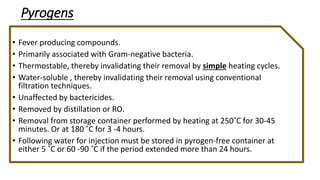
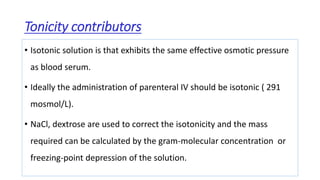
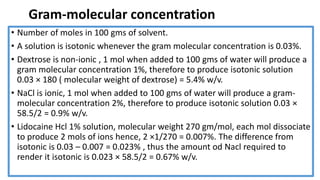
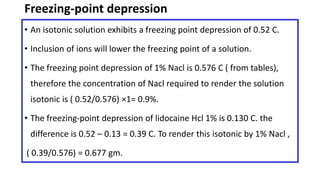
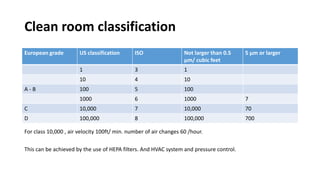
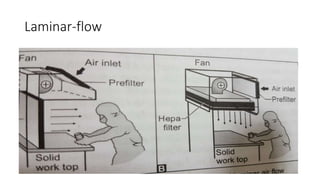
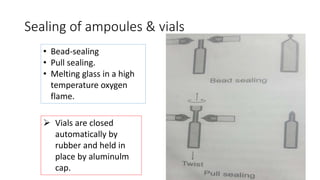
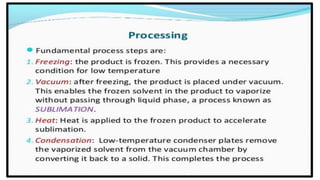
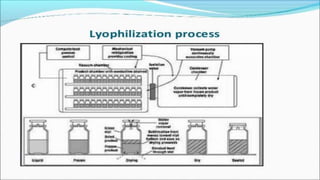
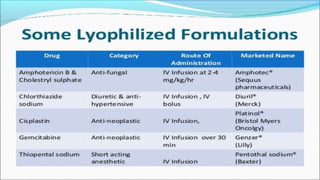
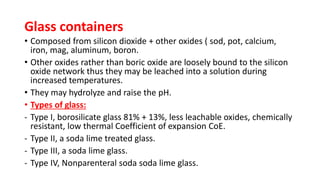
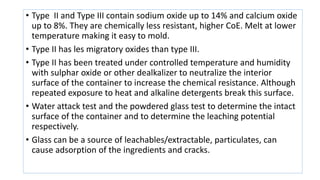
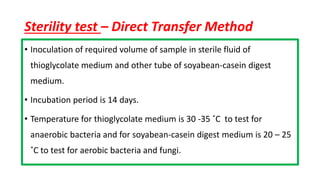
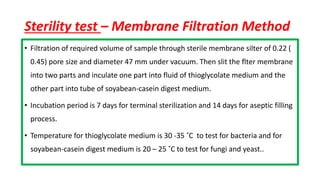
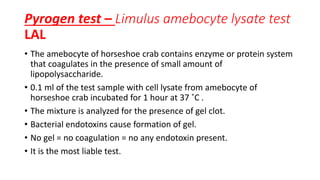
Parenteral products are sterile solutions, suspensions, or emulsions that are administered directly into the body by injection through various routes such as intravenous, intramuscular, or subcutaneous. They provide pure active ingredients free from contamination and immediate physiological effects. Parenteral formulations must consider factors like the drug's solubility, desired route of administration, dosage volume, and onset/duration of action. Common vehicles include water, isotonic saline solutions, and nonaqueous solvents. Additives like co-solvents and surfactants may also be included to aid formulation.