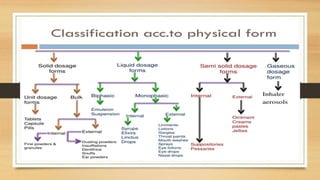
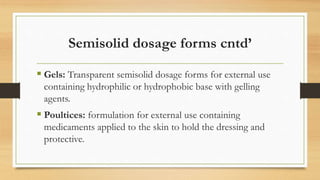
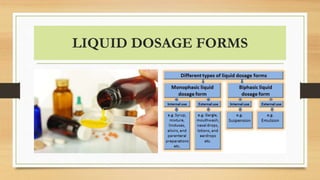
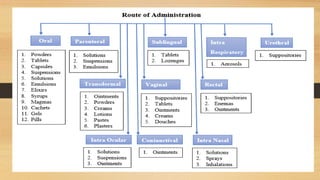
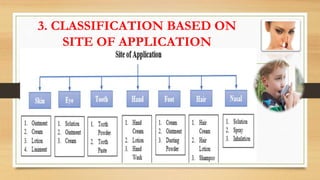
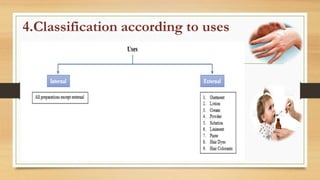
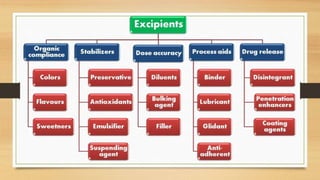
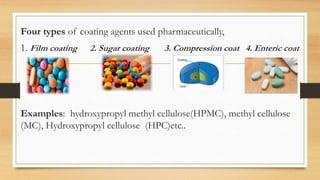
This document provides an introduction to pharmaceutical dosage forms and excipients. It begins by classifying dosage forms according to their physical state (solid, semi-solid, liquid, gaseous), route of administration, site of application, and therapeutic use. Examples are given for each classification. The document then discusses pharmaceutical excipients, their purposes and ideal properties. Common excipients like binders, diluents, disintegrants, lubricants and glidants are defined along with examples.