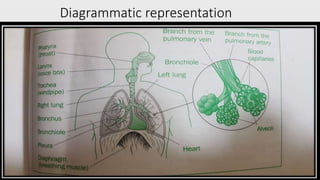
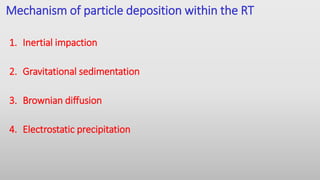
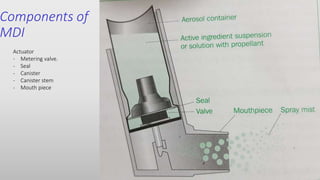
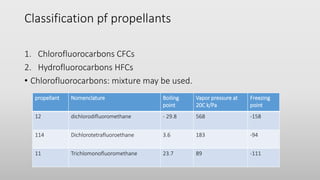
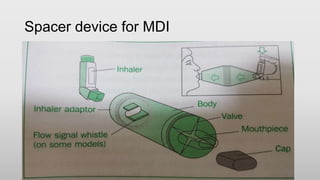
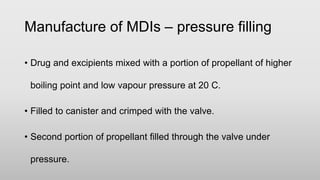
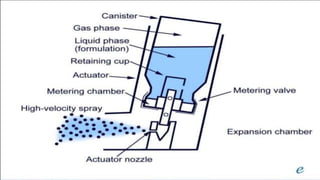
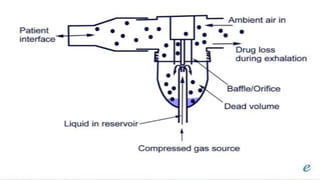
The document discusses respiratory dosage forms including their rationale, advantages, disadvantages, and formulation strategies. The main dosage forms are aerosols, dry powder inhalers, and nebulizers. Key points include that the dosage forms allow local or systemic drug delivery via the lungs. Factors like particle size and humidity affect deposition in the respiratory tract. Metered dose inhalers use propellants while dry powder inhalers do not. Formulations must consider factors like drug solubility, particle size, and buffering to ensure targeted delivery and patient safety.