1. The document discusses quality control tests for tablets, including tests for tablet characteristics before and after compression. It describes tests for properties like hardness, friability, thickness, weight variation, drug content uniformity, and dissolution.
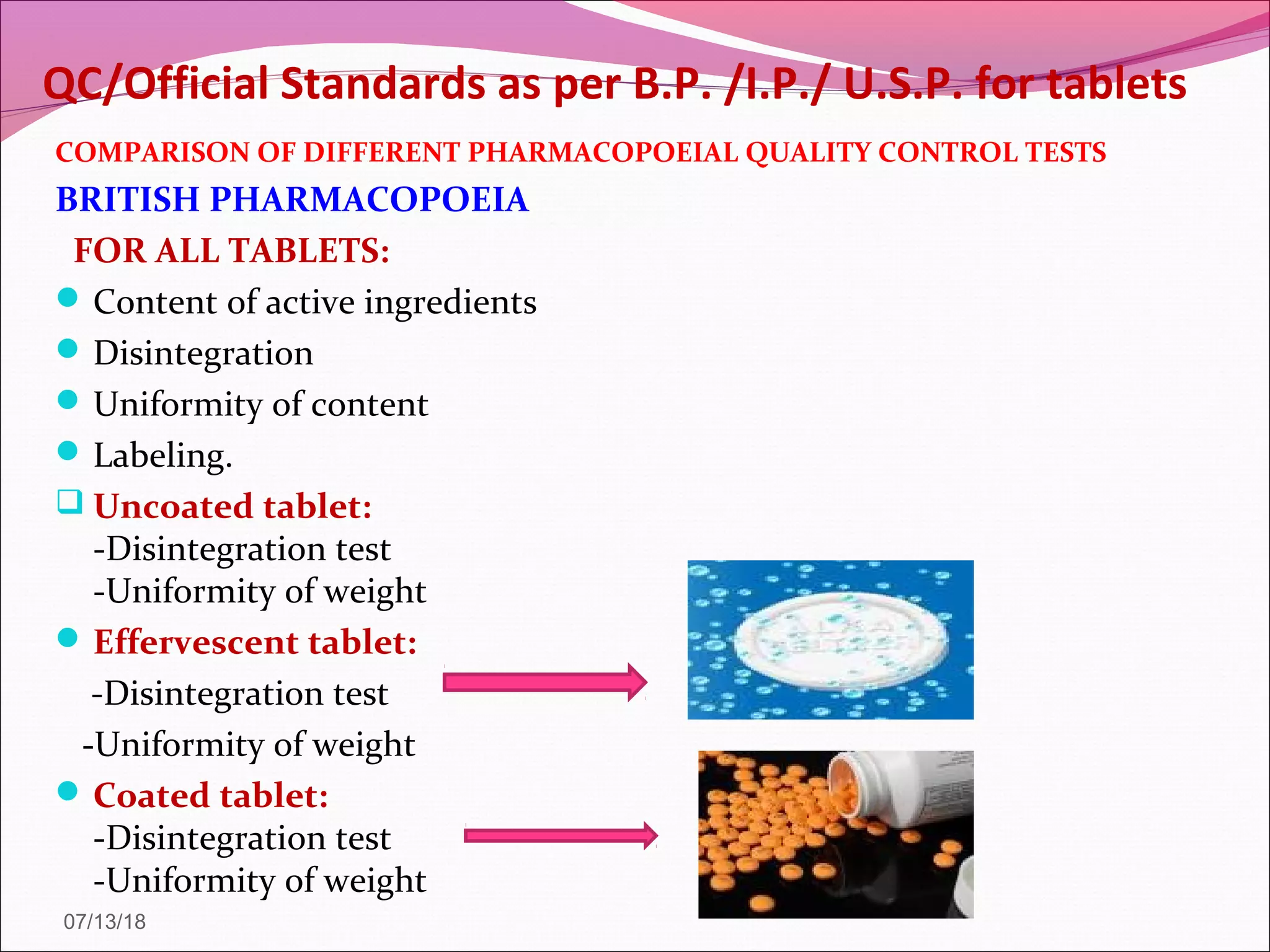
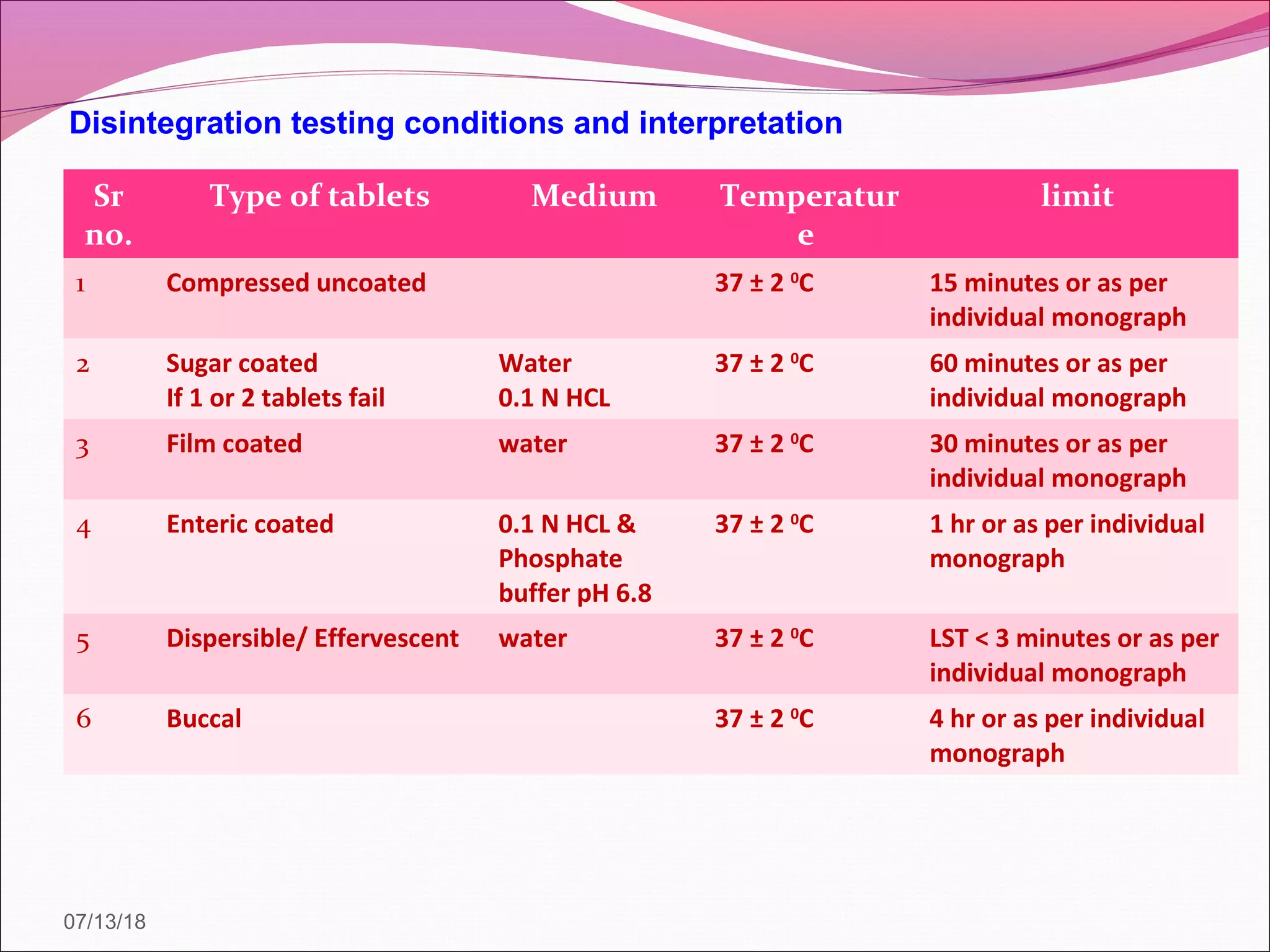
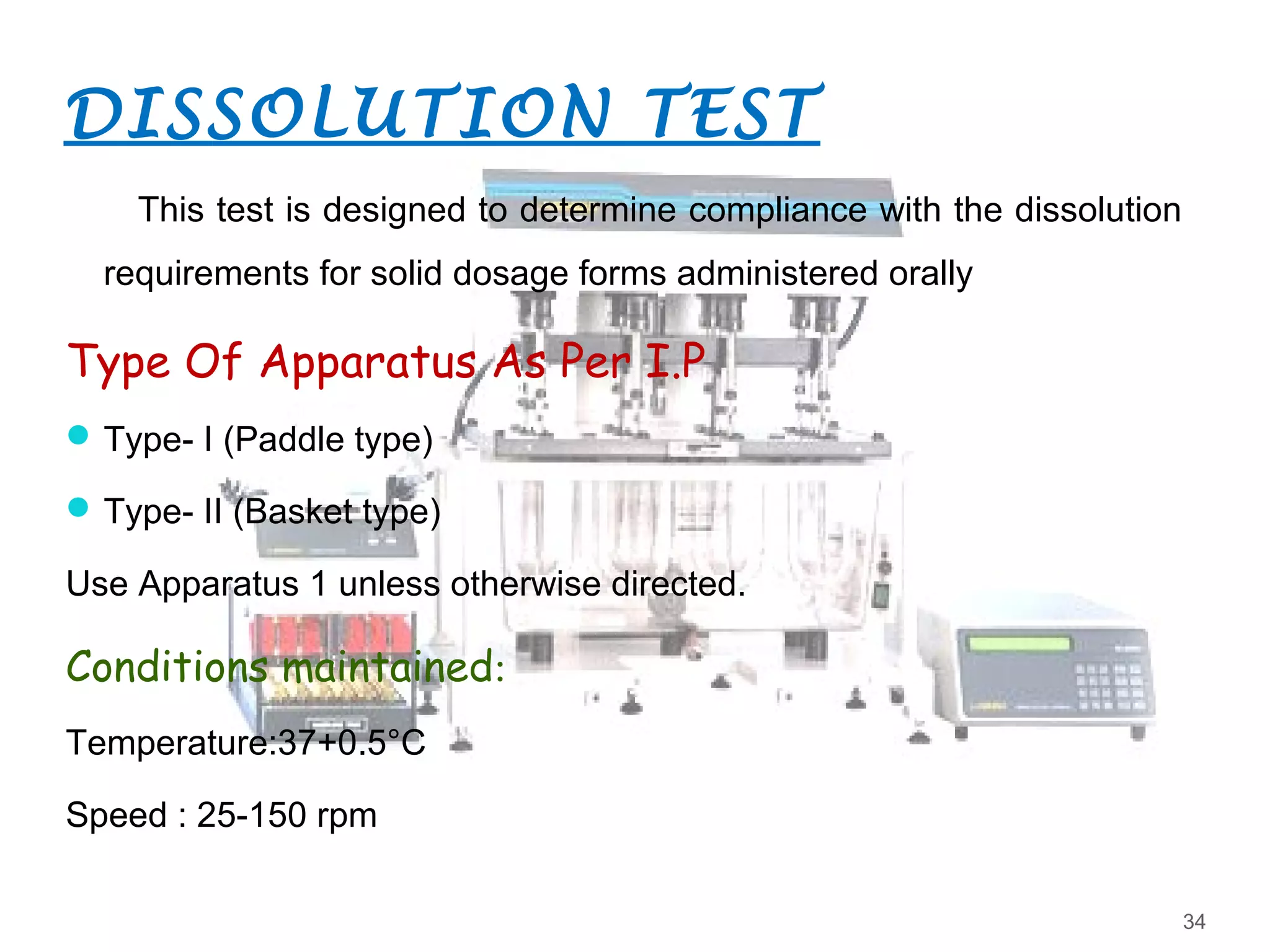
2. Official tests discussed include weight variation, disintegration, dissolution, and drug content/assay. Non-official tests include hardness and friability. Test conditions and acceptance criteria are provided for many of the tests.
3. The purpose of the quality control tests is to ensure tablets include the correct dose of drug, the drug is released in a controlled and reproducible manner, and the tablets have sufficient mechanical strength.