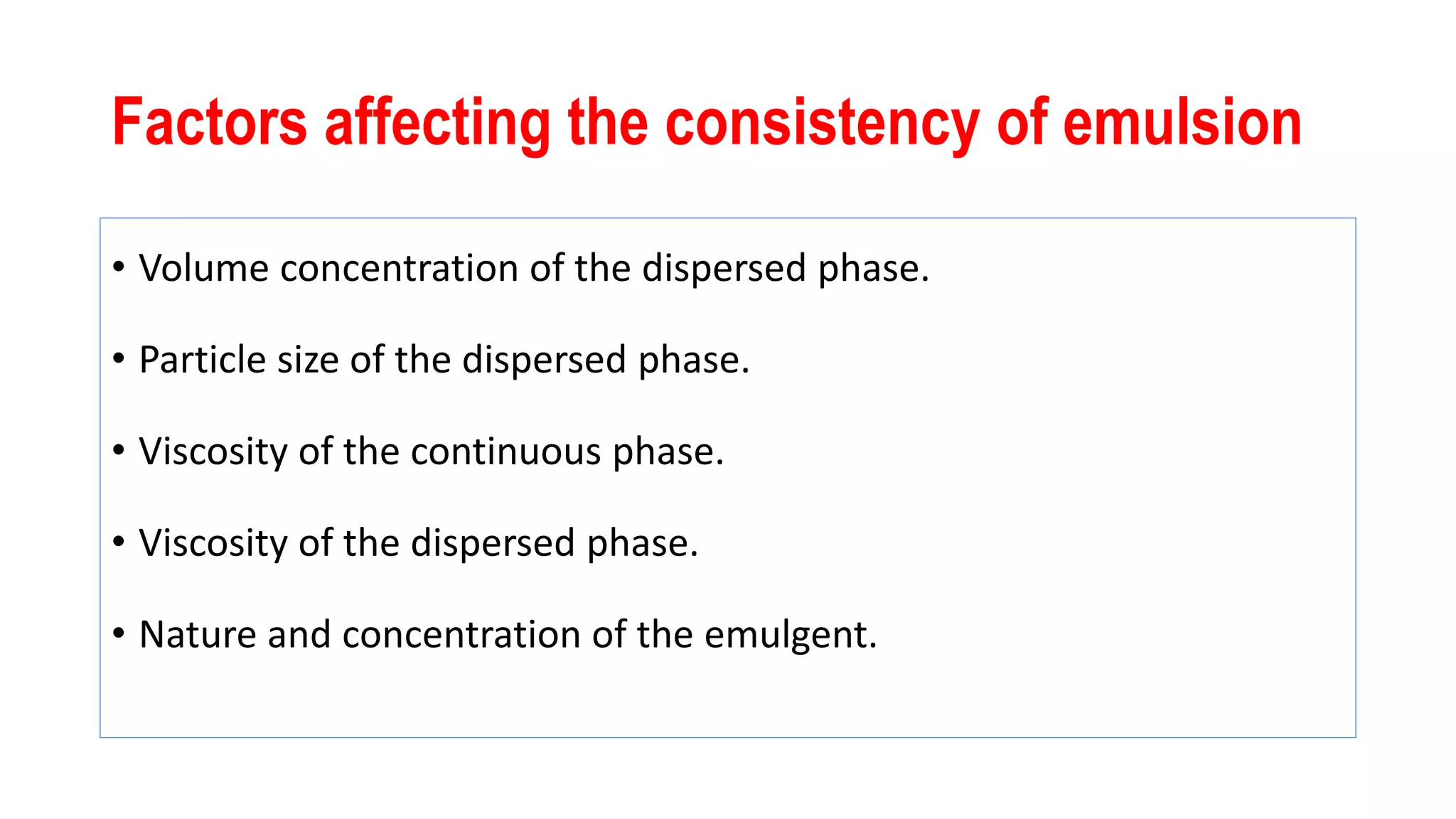
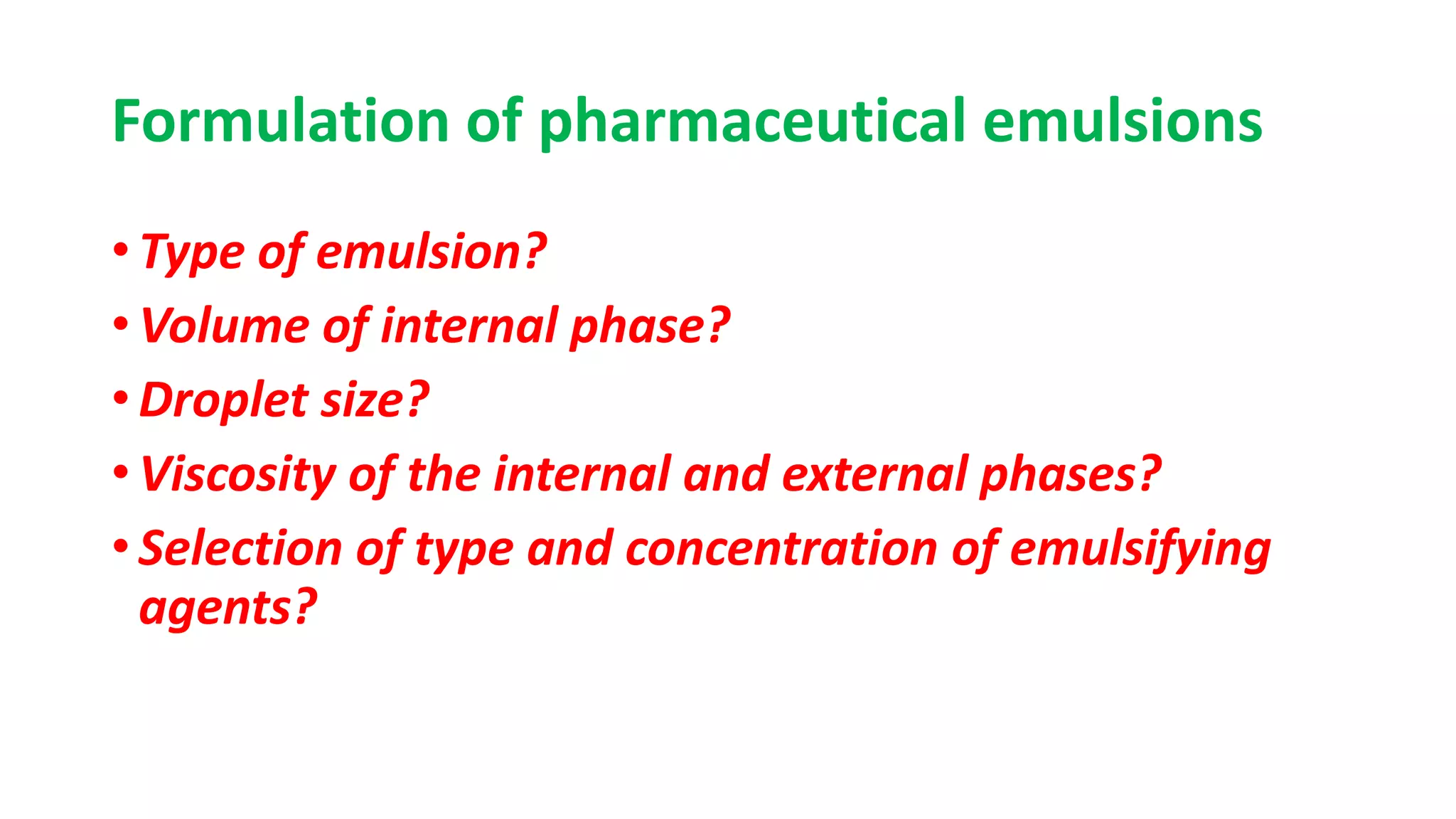
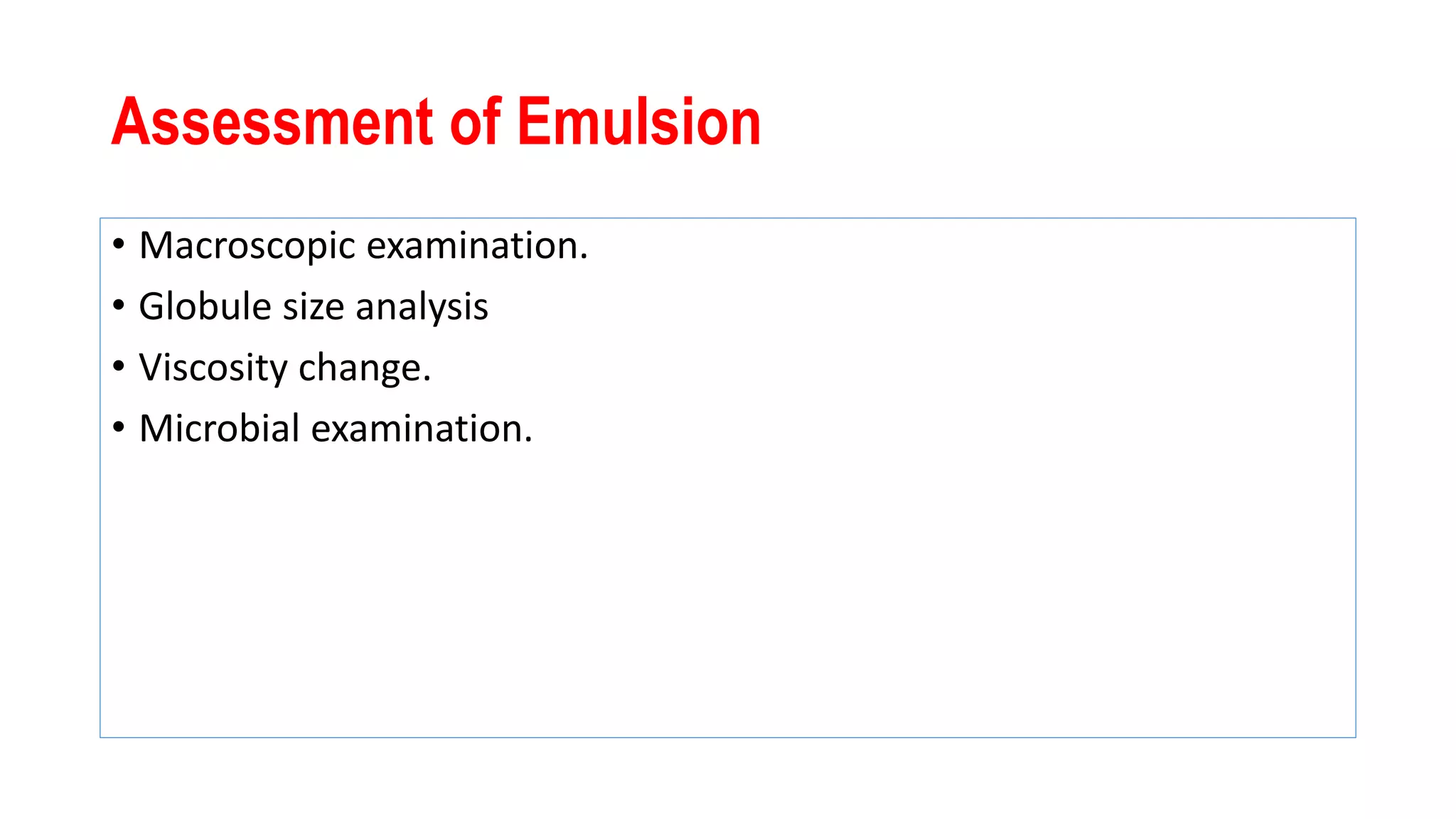
This document discusses liquid oral dosage forms, specifically oral solutions and suspensions. It provides details on the formulation, ingredients, advantages, and types of oral solutions and suspensions. Key points include:
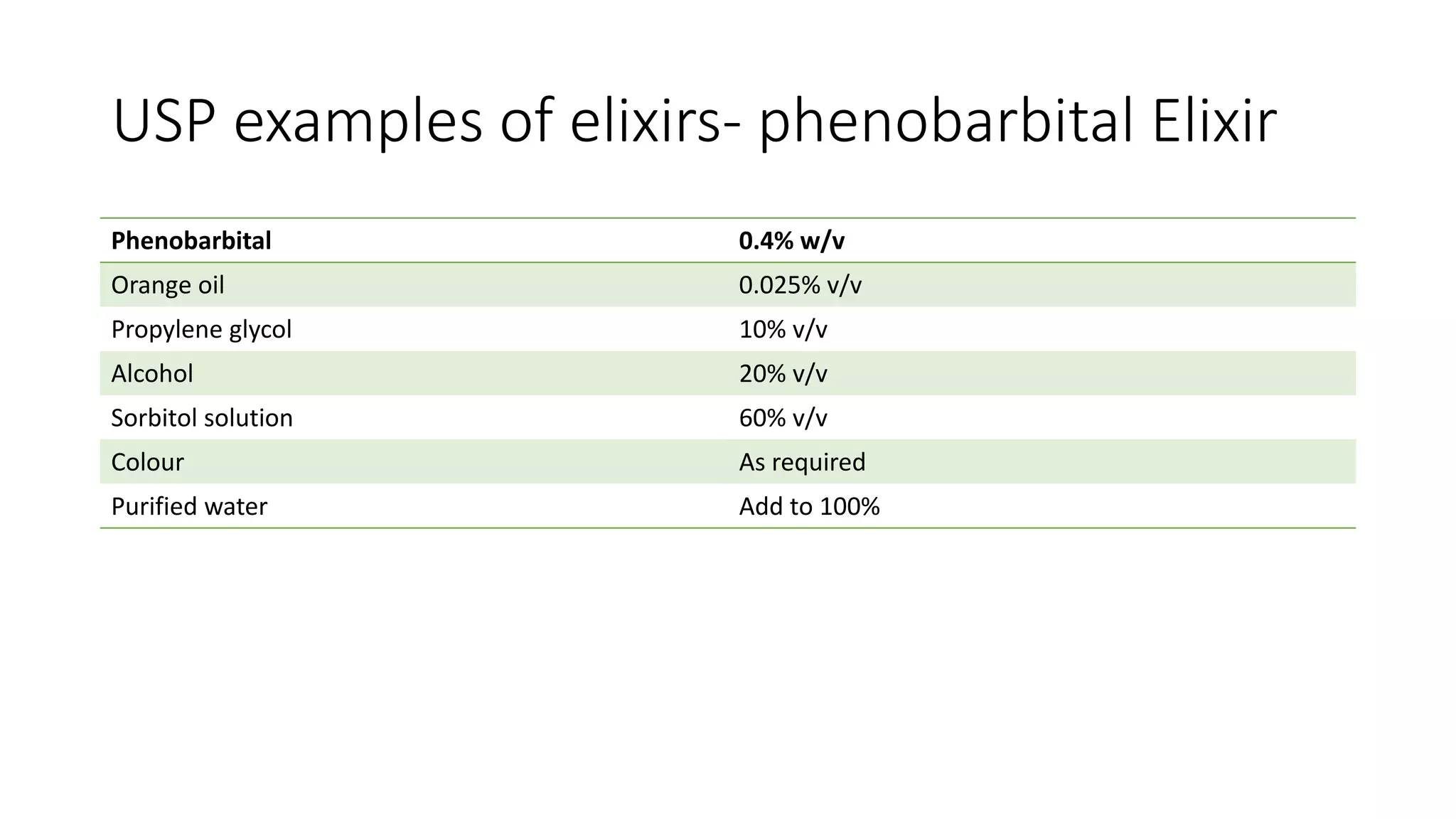
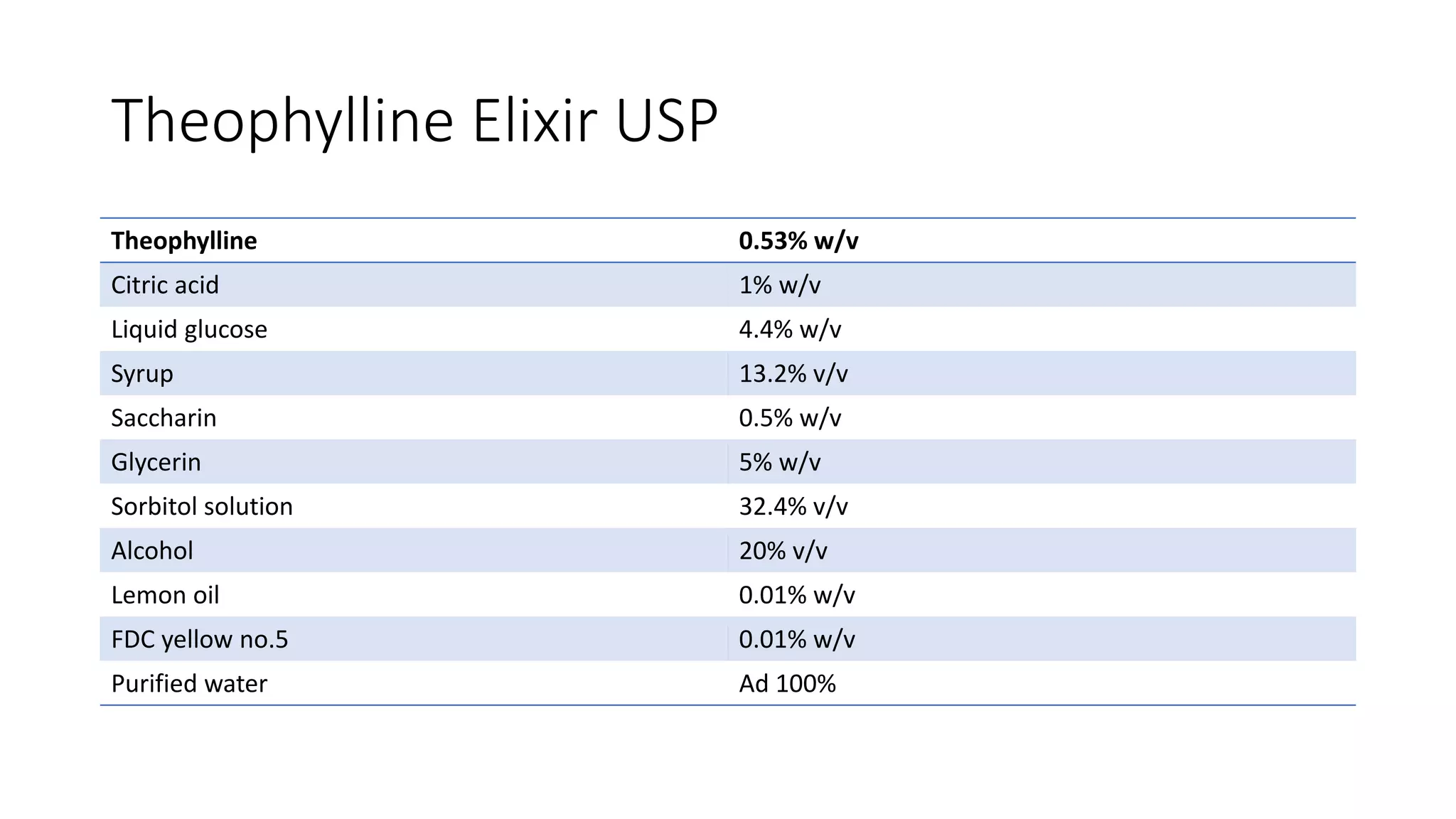
- Oral solutions are liquid preparations where the active ingredient and excipients are dissolved in a solvent system. Common types are oral solutions, syrups, elixirs, and mouthwashes.
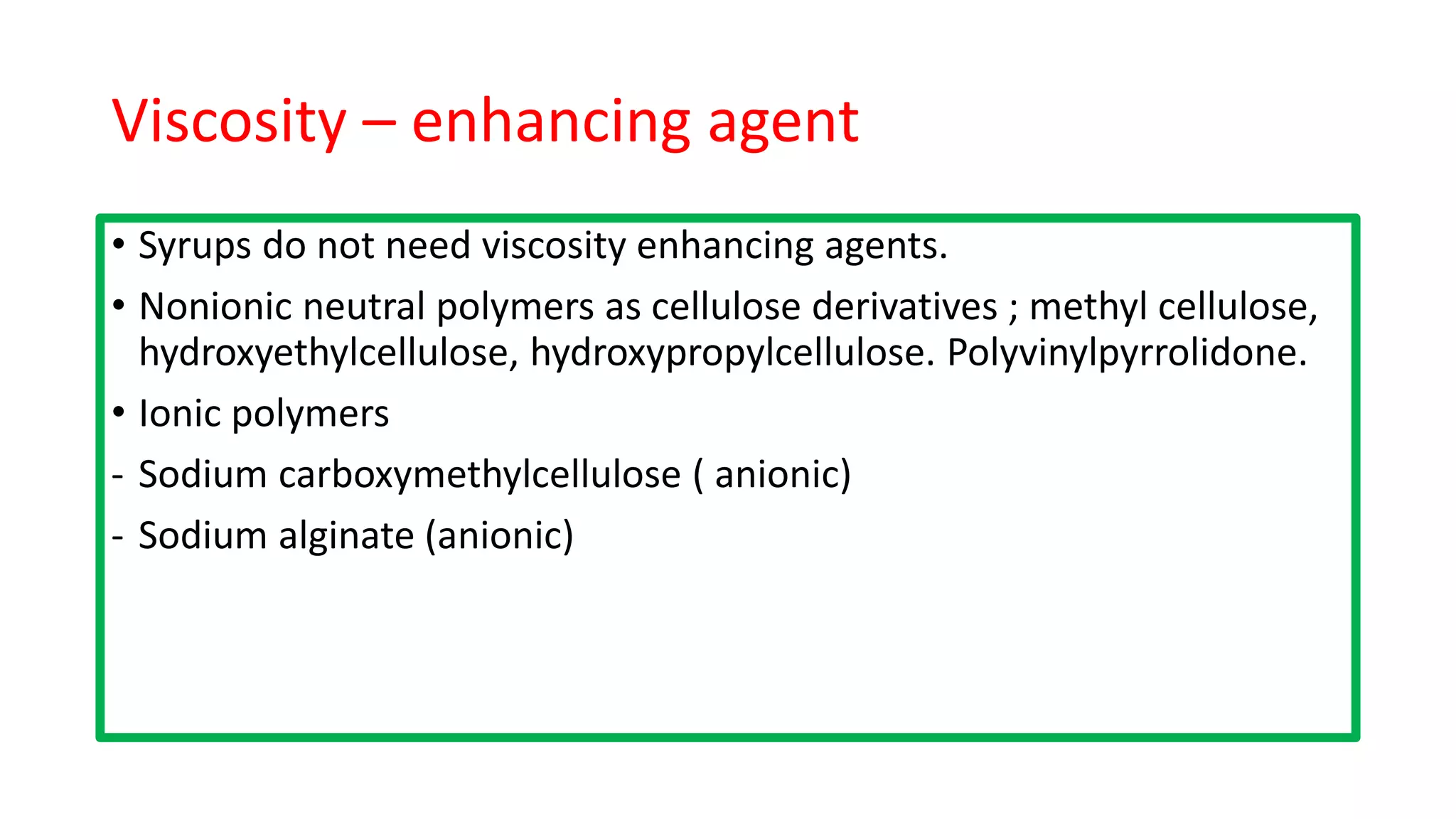
- Excipients in oral solutions include vehicles, co-solvents, surfactants, preservatives, sweeteners, and viscosity modifiers. Water is a common vehicle and glycerol, alcohols, and propylene glycol are used as co-solvents.
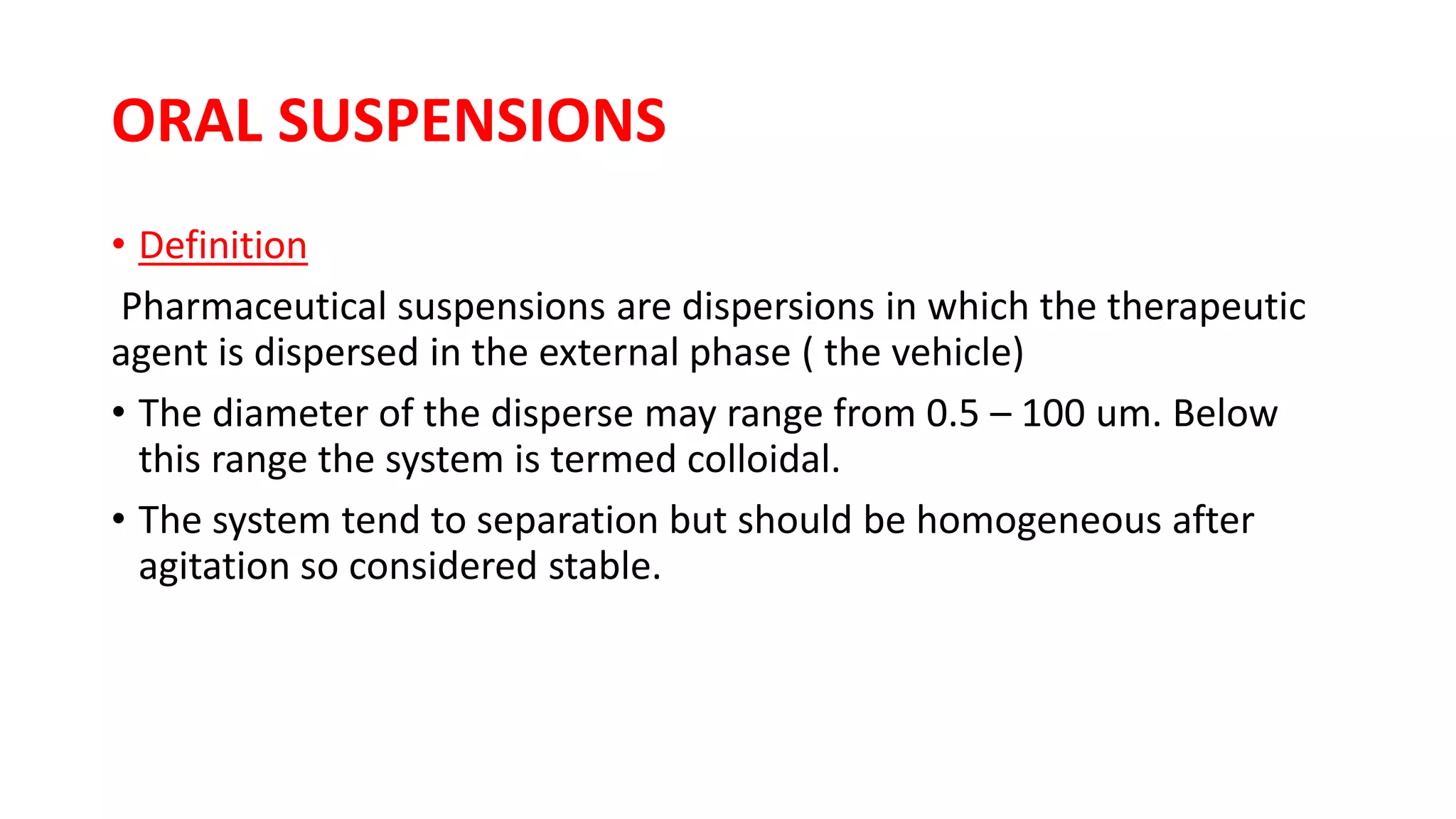
- Oral suspensions are dispers