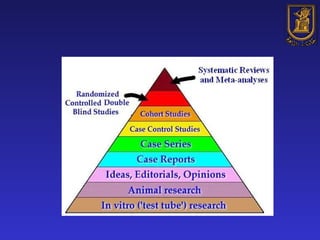
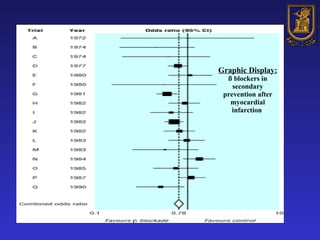
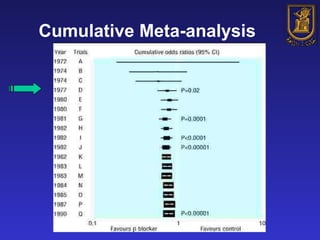
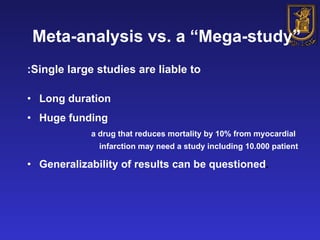
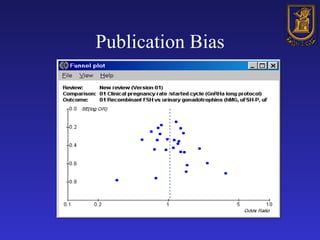
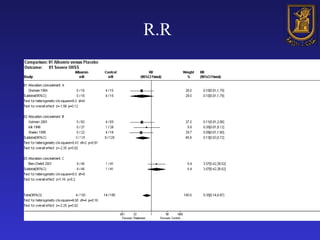
A systematic review is a structured review that pools the results of multiple studies on a topic using meta-analysis. It aims to summarize evidence from studies addressing a specific clinical question in a rigorous, unbiased manner to explain differences among studies on the same question. Systematic reviews are considered the highest level of evidence and are needed because individual studies may have biases or low statistical power to detect effects.