Uterine sarcoma is a rare and challenging type of cancer that grows rapidly. It accounts for 2-5% of uterine malignancies and is diagnosed in about 17 per 1000 women annually. Risk factors include prior pelvic radiation and black race. Long-term tamoxifen use also increases the risk. The most common presenting symptom is vaginal bleeding. Surgery is the primary treatment but the benefits of adjuvant radiation and chemotherapy are unclear due to limited data. Prognosis is generally poor, especially for later stages, and more research is needed to determine optimal adjuvant therapies.


![Epidemiology Rare 2% to 5% of all uterine malignancies 17 per million women annually [Platz, & Benda, 1995] Between 1989-1999, 2677 women were diagnosed with uterine sarcoma (Brooks et al, April, 2004)](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-3-320.jpg)

![long-term adjuvant tamoxifen An increase in the risk of uterine sarcomas appears to accompany the use of long-term adjuvant tamoxifen in women with breast cancer [Wickerham et al, 2002, Wysowski et al, 2002]. Since 1978, when tamoxifen was first marketed in the United States, 159 cases of uterine sarcoma worldwide have been reported](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-5-320.jpg)
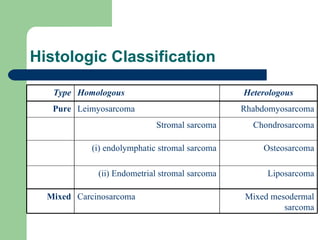

![Leiomyosarcoma Arise from smooth muscles of the uterus usually de novo appear grossly as a large (>10 cm) yellow or tan solitary mass with soft, fleshy cut surfaces exhibiting hemorrhage and necrosis [Viereck et al, 2002].](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-8-320.jpg)

![Leiomyosarcoma: Low or high grade Frequent mitotic figures significant nuclear atypia, presence of coagulative necrosis of tumor cells. [ Bell et al, 1994 ]](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-10-320.jpg)
![Endometrial stromal Sarcoma : A pure homologous neoplasm Subtypes: low and high grade Low grade : slow growing tumors with infrequent metastasis or recurrence after therapy. [Oliva, et al, 2000]. high grade : enlarge and metastasize quickly and are often fatal.](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-11-320.jpg)





![Rapidly growing!! Among 341 women with a rapidly growing uterus by clinical or ultrasound examination, only one (0.27 percent) had a uterine sarcoma . [Parker et al, 1994].](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-17-320.jpg)
![Should be considered in postmenopausal women with a pelvic mass, abnormal bleeding, and pelvic pain, where the incidence of sarcoma is 1 to 2 percent [Leibsohn et al, 1990]](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-18-320.jpg)
![Evaluation Ultrasound examination, MRI, or CT scan cannot reliably distinguish between a sarcoma, leiomyoma or endometrial cancer [Rha et al, 2003]. The diagnosis of uterine sarcomas is made from histologic examination of the entire uterus](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-19-320.jpg)

![Lymph node Biopsy patients with uterine sarcoma grossly confined to the uterus/cervix showed lymph node metastases in 5 of 101 patients should be reserved for women with clinically suspicious nodes [Leitao et al, 2003 ]](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-21-320.jpg)
![Further support In one series of 208 women with uterine leiomyosarcoma, only four of 36 who underwent lymph node sampling had positive nodes [Giuntoli et al, 2003].](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-22-320.jpg)

![Surgery is the only curative therapy for uterine sarcomas [Morice et al, 2003]](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-24-320.jpg)



![three-year local recurrence rates No adjuvant treatment 62 % Whole pelvis external beam radiation therapy 31 % Chemotherapy alone 71 percent [Livi et al, 2003]](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-28-320.jpg)
![Massachusetts General Hospital 1990-1999 Adjuvant therapy after optimal cytoreduction does not decrease the rate of recurrence [Dinh et al, 2004]](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-29-320.jpg)
![Adjuvant radiation therapy Some studies of postoperative radiation suggest a survival benefit [Moskovic et al, 1993 Knocke et al, 1998, Weitmann et al, 2001]. Other studies showed cure rate was similar for those treated with surgery alone or followed by radiation, regardless of the stage of disease [Giuntoli et al, 2003]](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-30-320.jpg)



![Leiomyosarcoma doxorubicin is an effective drug for advanced leiomyosarcoma combinations with doxorubicin increase the objective response rate but add substantial toxicity A very recent small trial showed promising results with gemcitabine plus docetaxel [Hensley et al, 2002].](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-34-320.jpg)
![Carcinosarcoma benefit from cisplatin-based chemotherapy particularly combinations of cisplatin with doxorubicin and ifosfamide, or single agent paclitaxel [ Gallup et al, 2003 , van Rijswijk et al, 2003, Harris et al, 2003]](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-35-320.jpg)
![Mixed mesodermal tumors Cisplatin and ifosfamide appear to have greater activity than does doxorubicin alone [Ramondetta et al, 2003]. In a very small uncontrolled trial : cisplatin, doxorubicin, and dacarbazine give three year survivals of 51 % [Baker et al, 1991].](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-36-320.jpg)


![In a recent RCT 2000 ifosfamide with or without cisplatin for recurrent sarcoma demonstrated a higher response rate on the combination arm However,use of the combination was not justified because of increased toxic effects [Sutton et al, 2000]](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-39-320.jpg)
![Prognosis poor prognosis the 5-year survival : stage I less than 50% remaining stages : 0% to 20%. strongest predictor of survival was menopausal status at time of diagnosis [Major et al, 1993]](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-40-320.jpg)
![leiomyosarcoma age over 50 years was a poor prognostic factor, as was size greater than 5 cm [Giuntoli et al, 2003].](https://image.slidesharecdn.com/uterinesarcoma-100612061146-phpapp01/85/Uterine-sarcoma-41-320.jpg)

