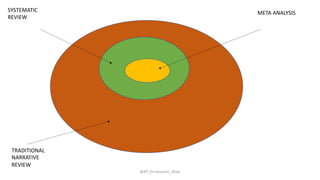
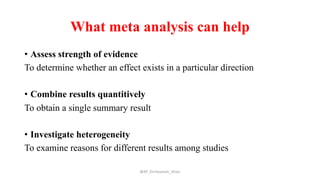
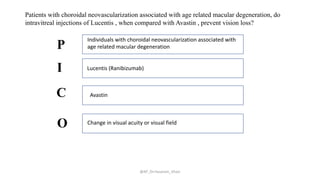
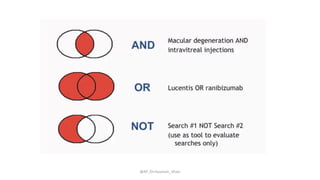
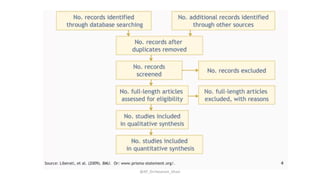
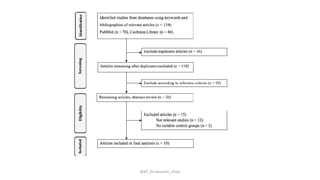
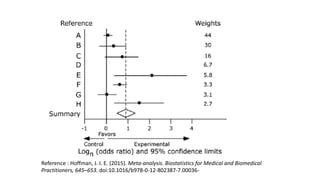
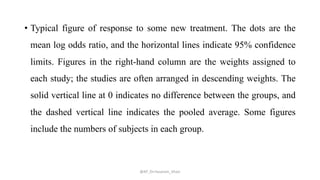
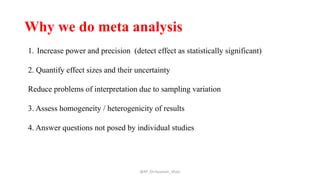
The document discusses systematic reviews and meta-analyses. It defines systematic reviews as a summary of available healthcare studies that provides high-level evidence on healthcare interventions. Meta-analyses use statistical methods to quantitatively summarize results across multiple studies. The document outlines the steps in conducting systematic reviews, including developing a protocol, searching for evidence, assessing risk of bias, and synthesizing findings. It also discusses how meta-analyses can help determine the strength and consistency of effects across studies.