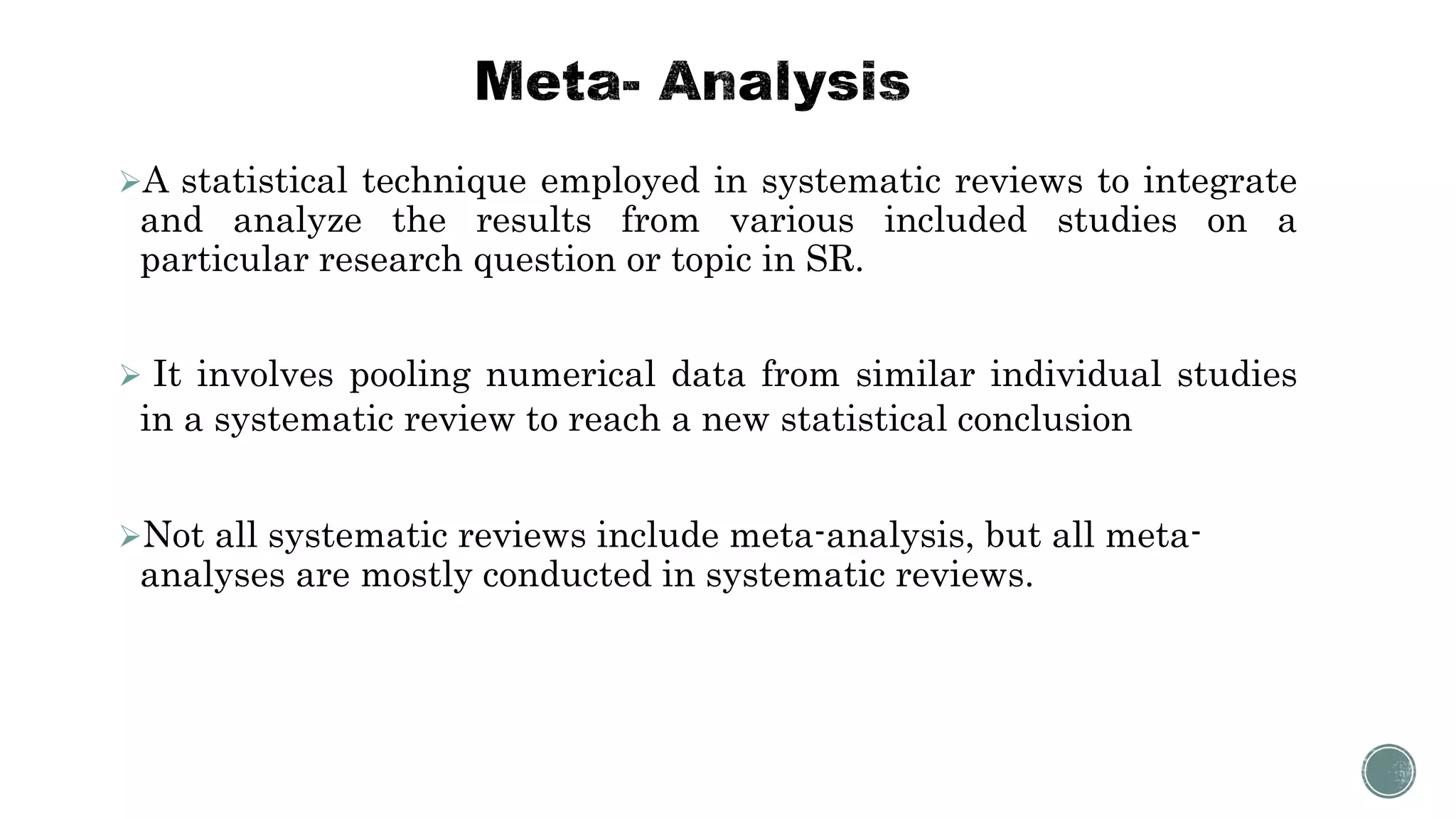
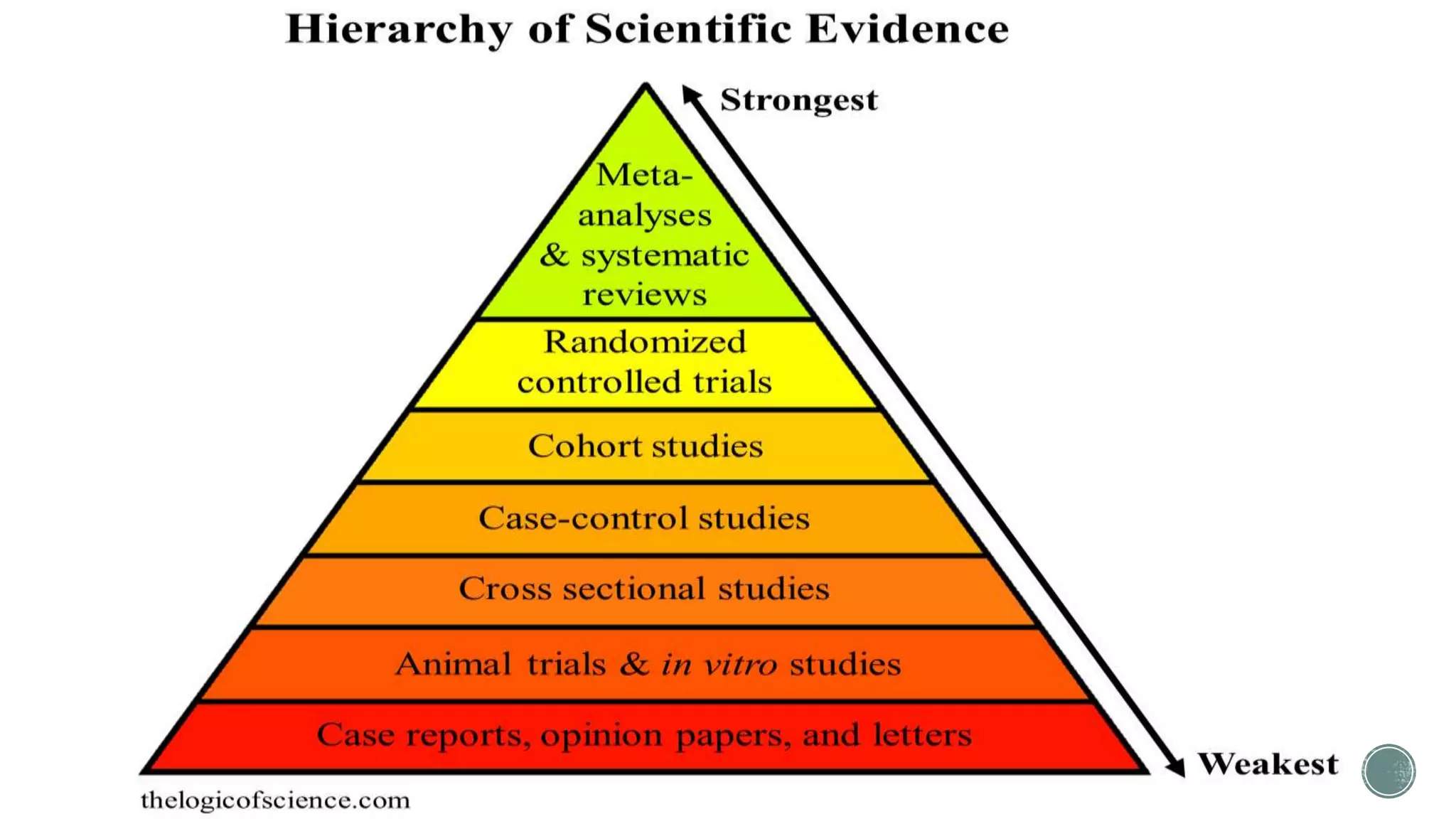
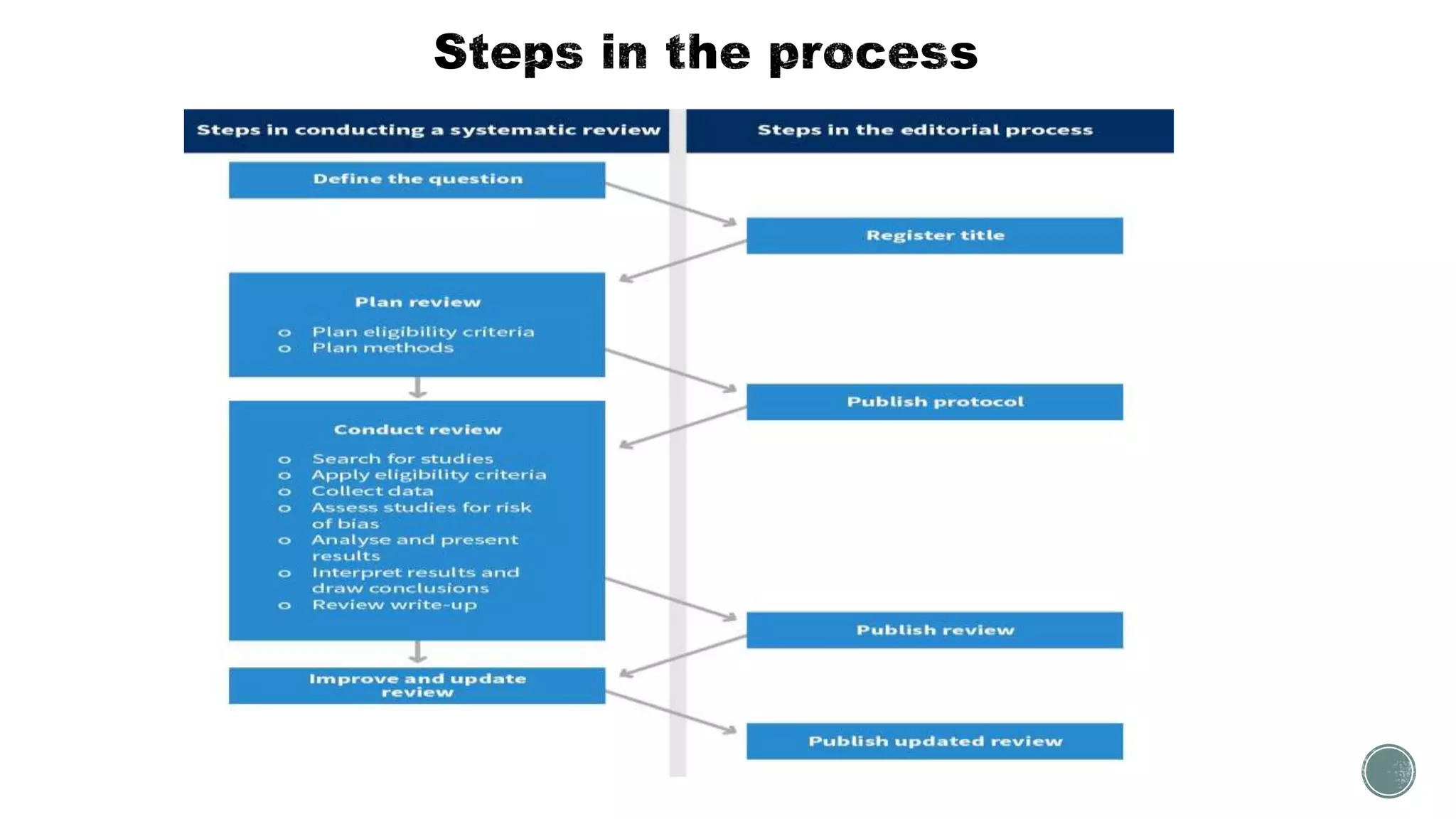
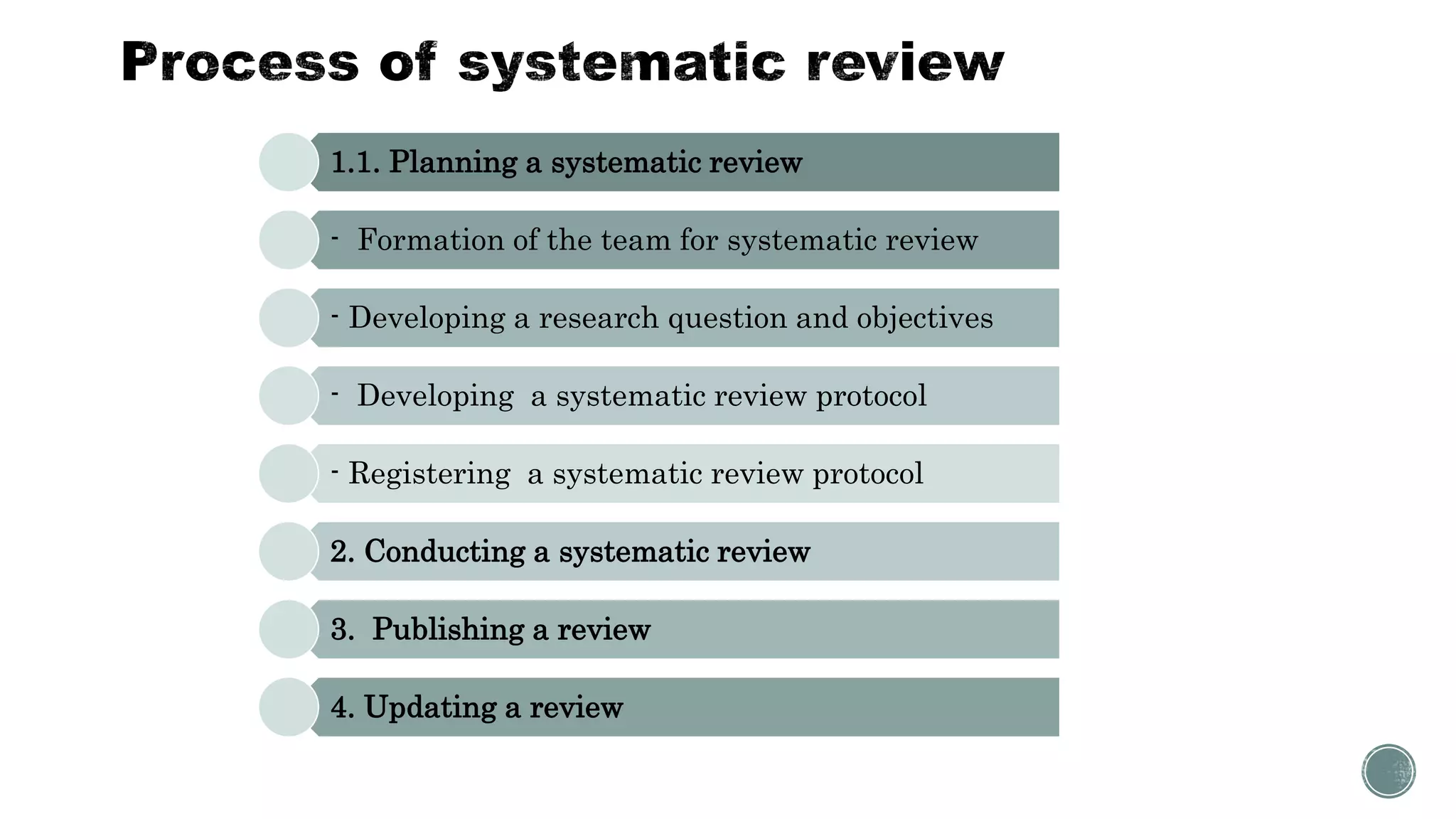
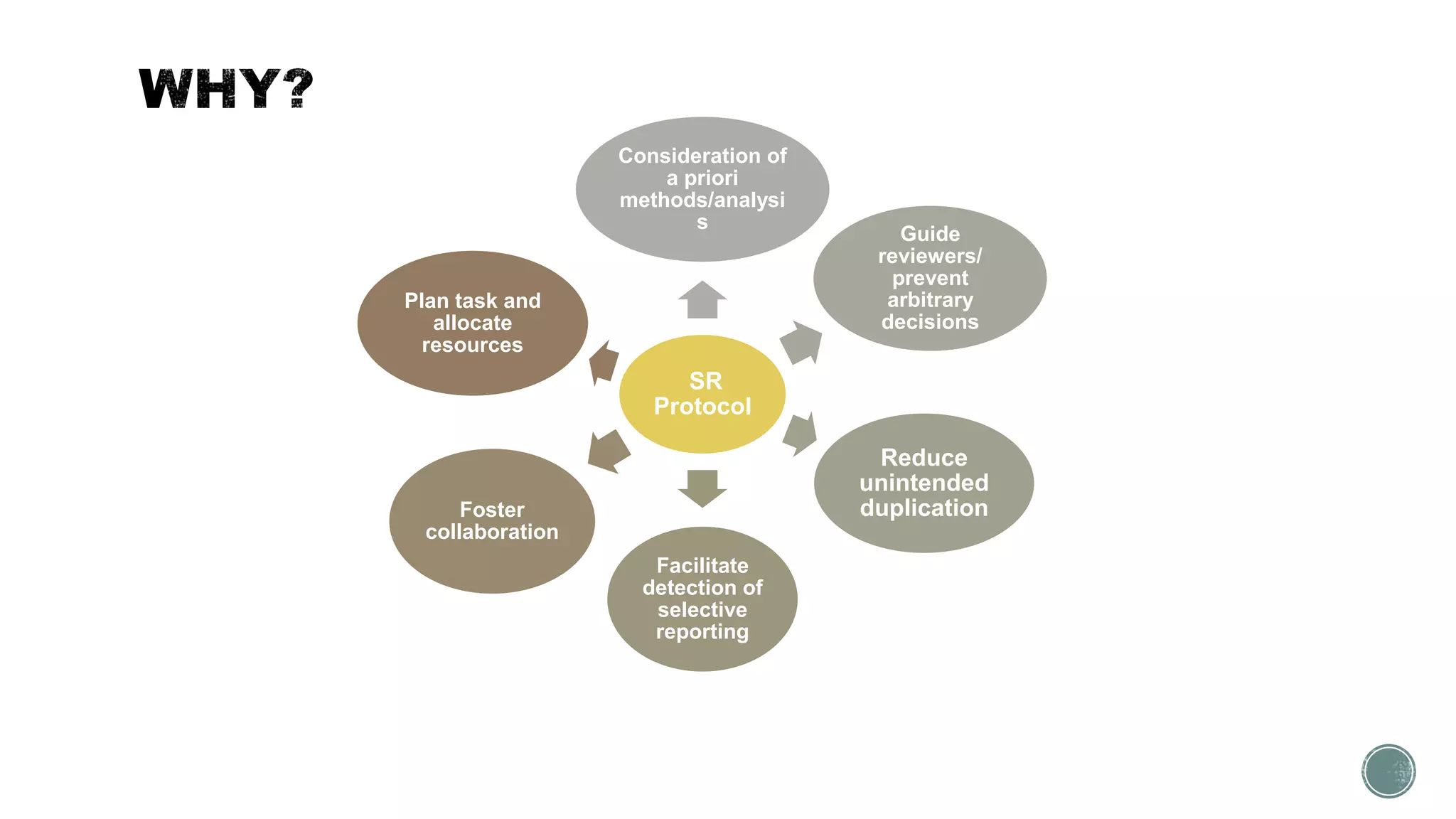
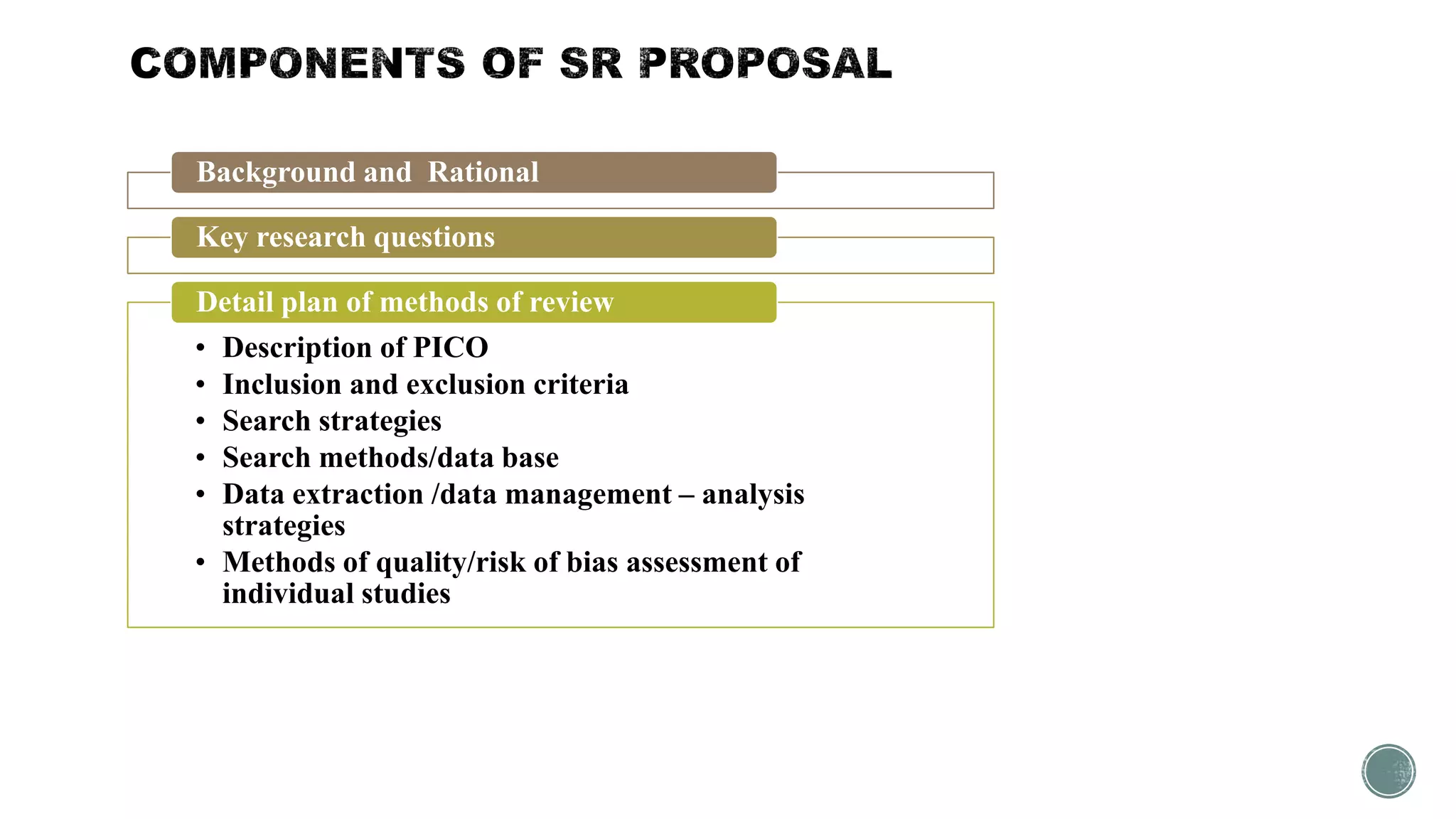
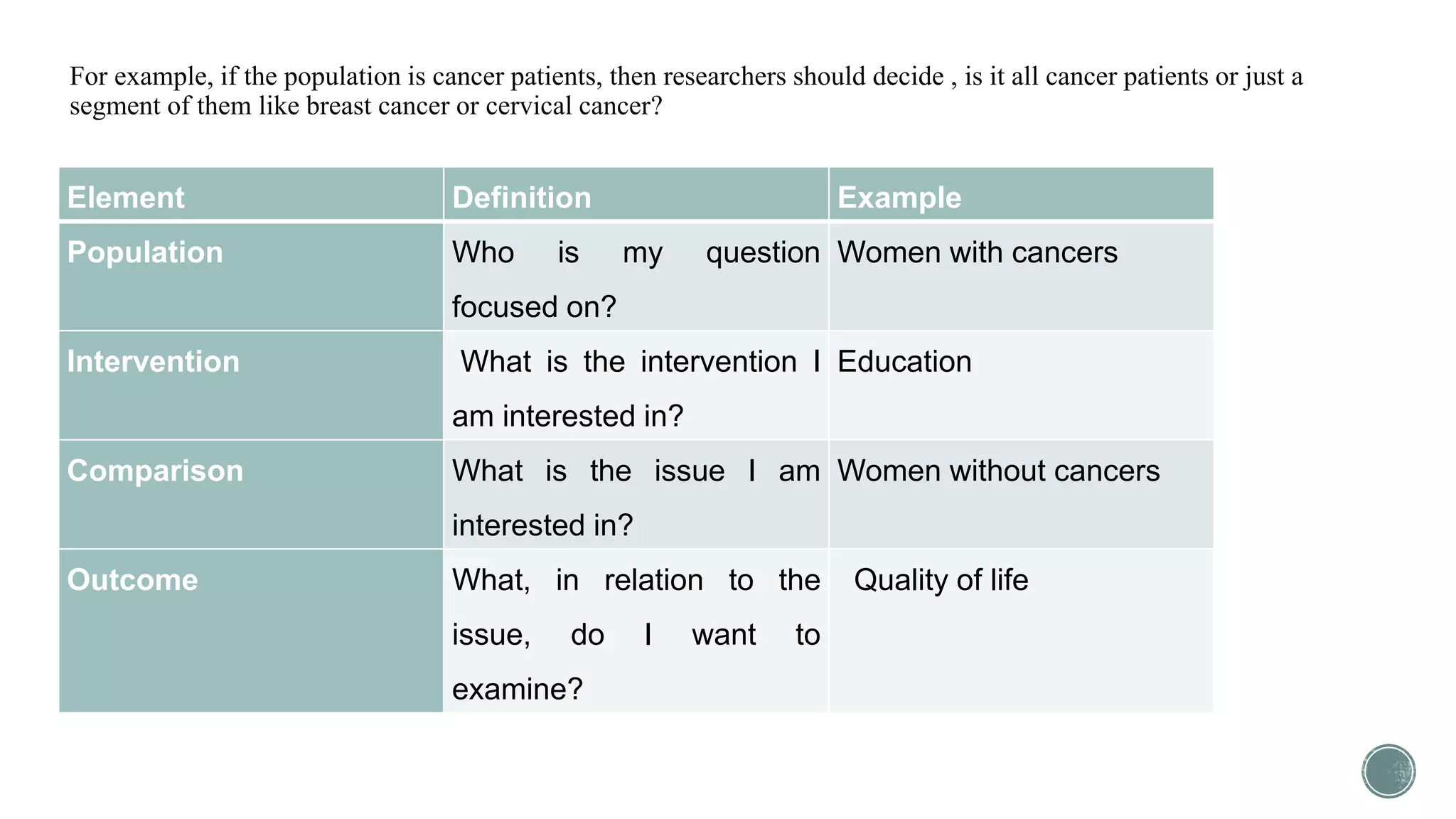
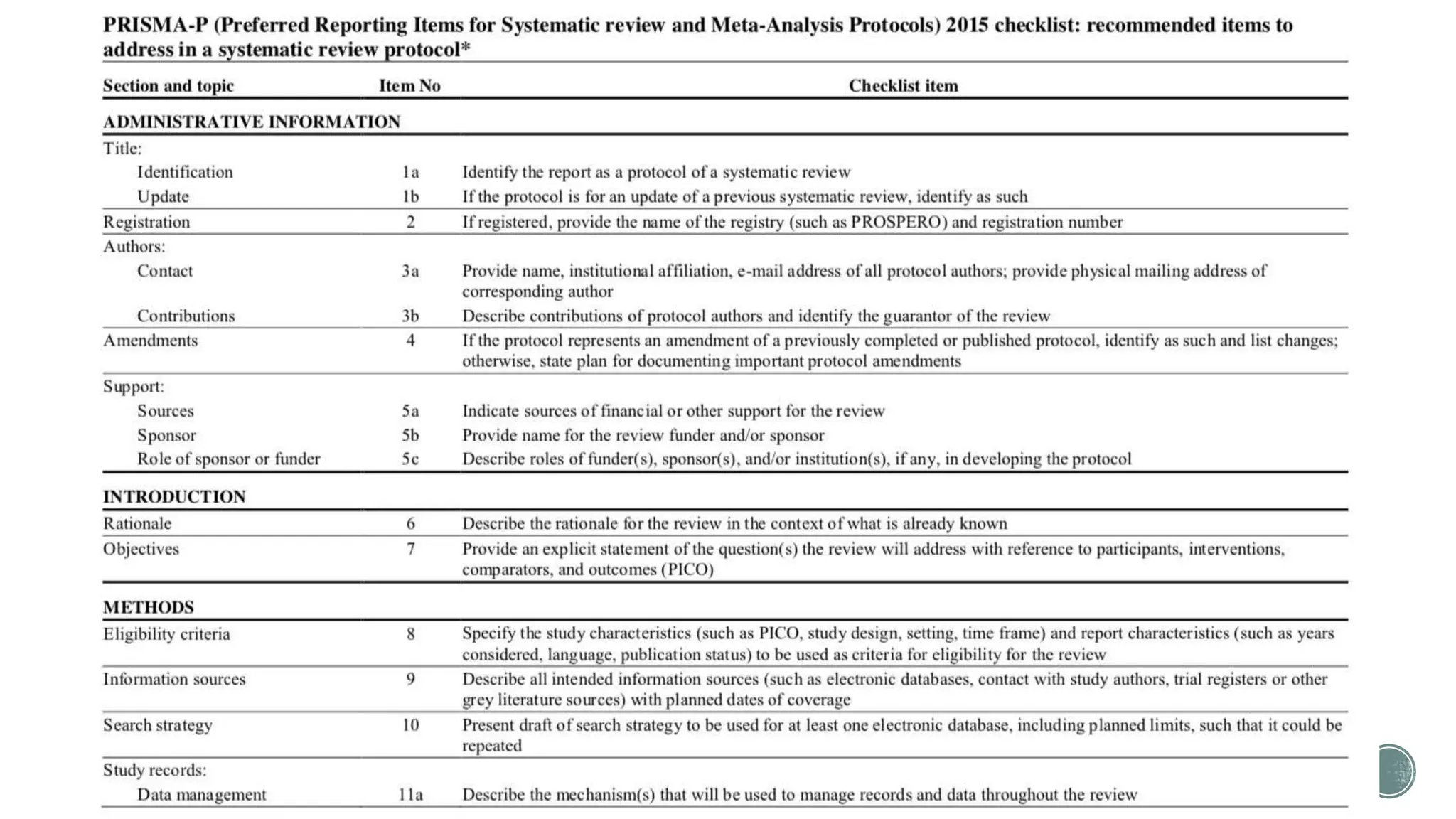
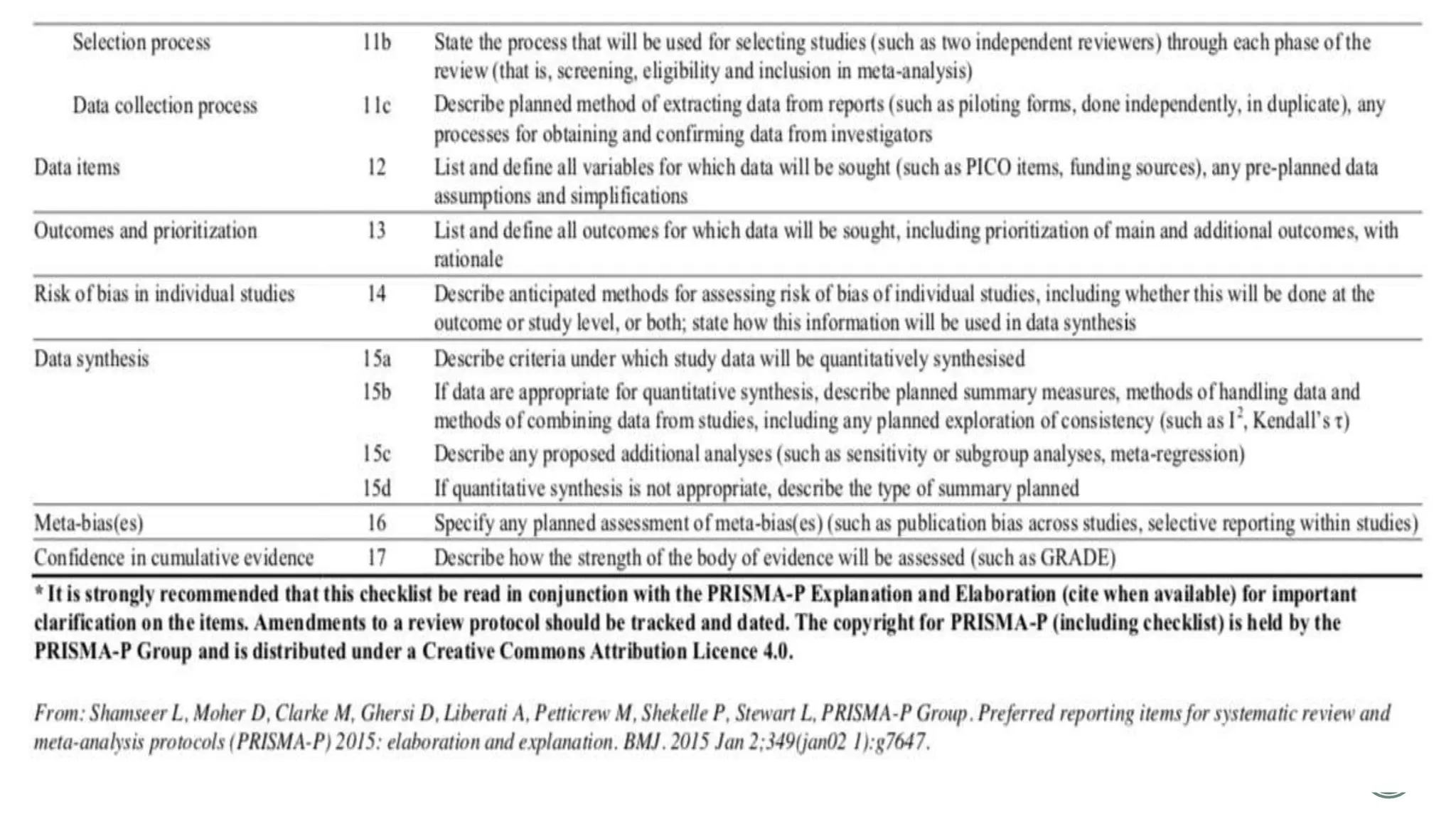
The document outlines the process of conducting systematic reviews, emphasizing the importance of a structured approach to synthesize evidence from multiple studies. Key steps include planning, developing a protocol, registering the review, and conducting rigorous data analysis with appropriate methodologies and tools. It also discusses the formation of a skilled research team and the need for thorough literature background checks to ensure the review addresses existing gaps.