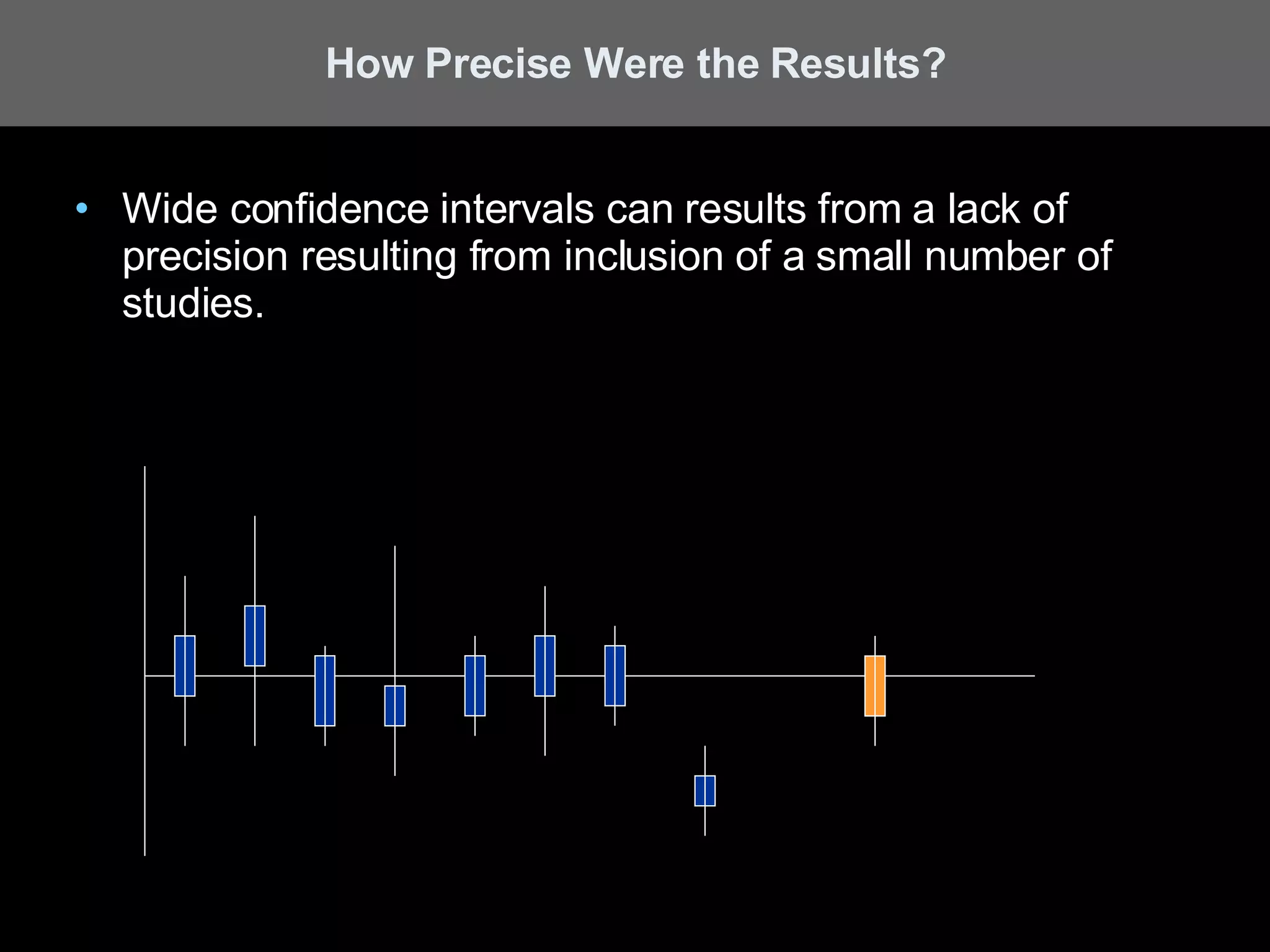
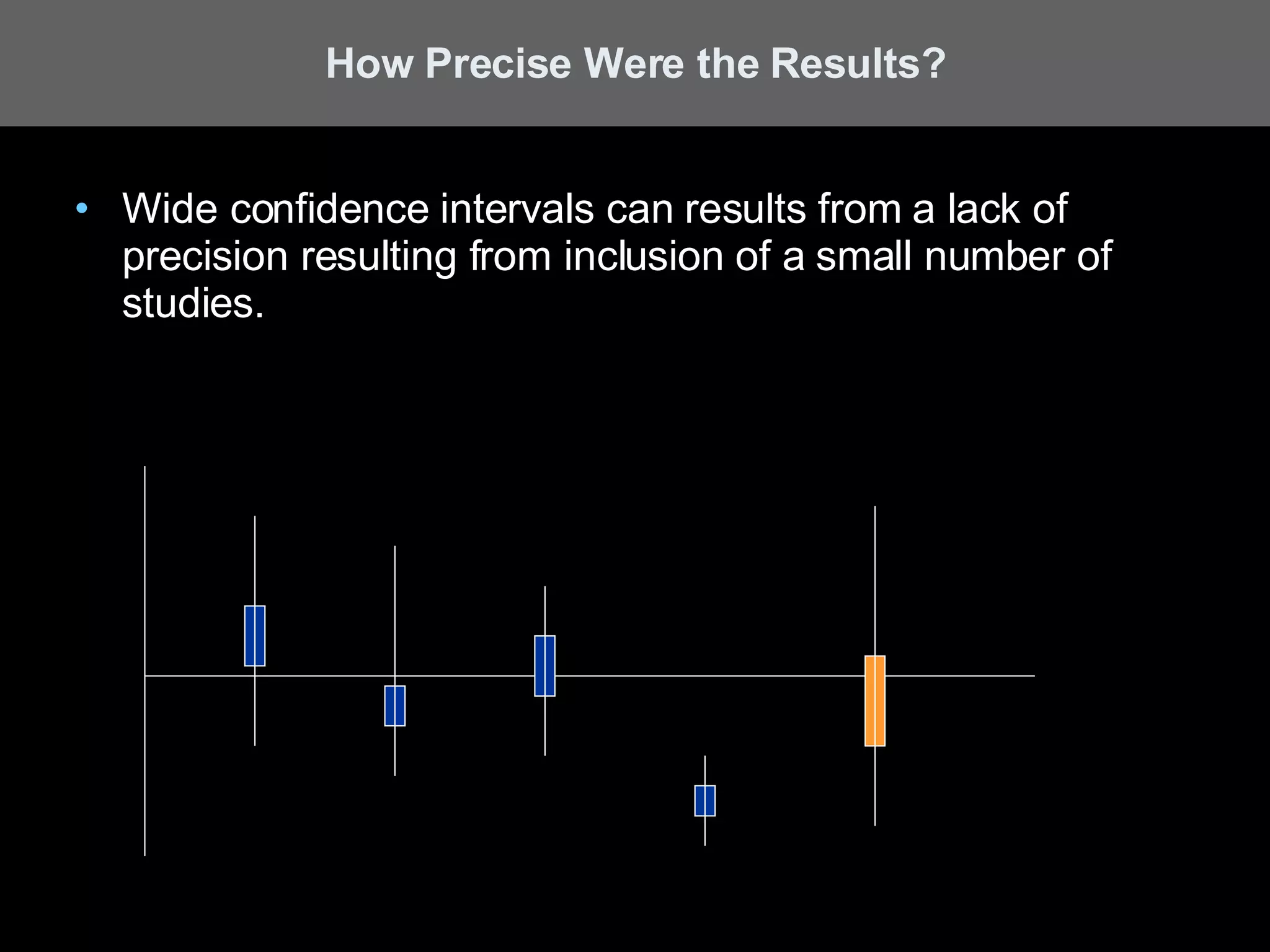
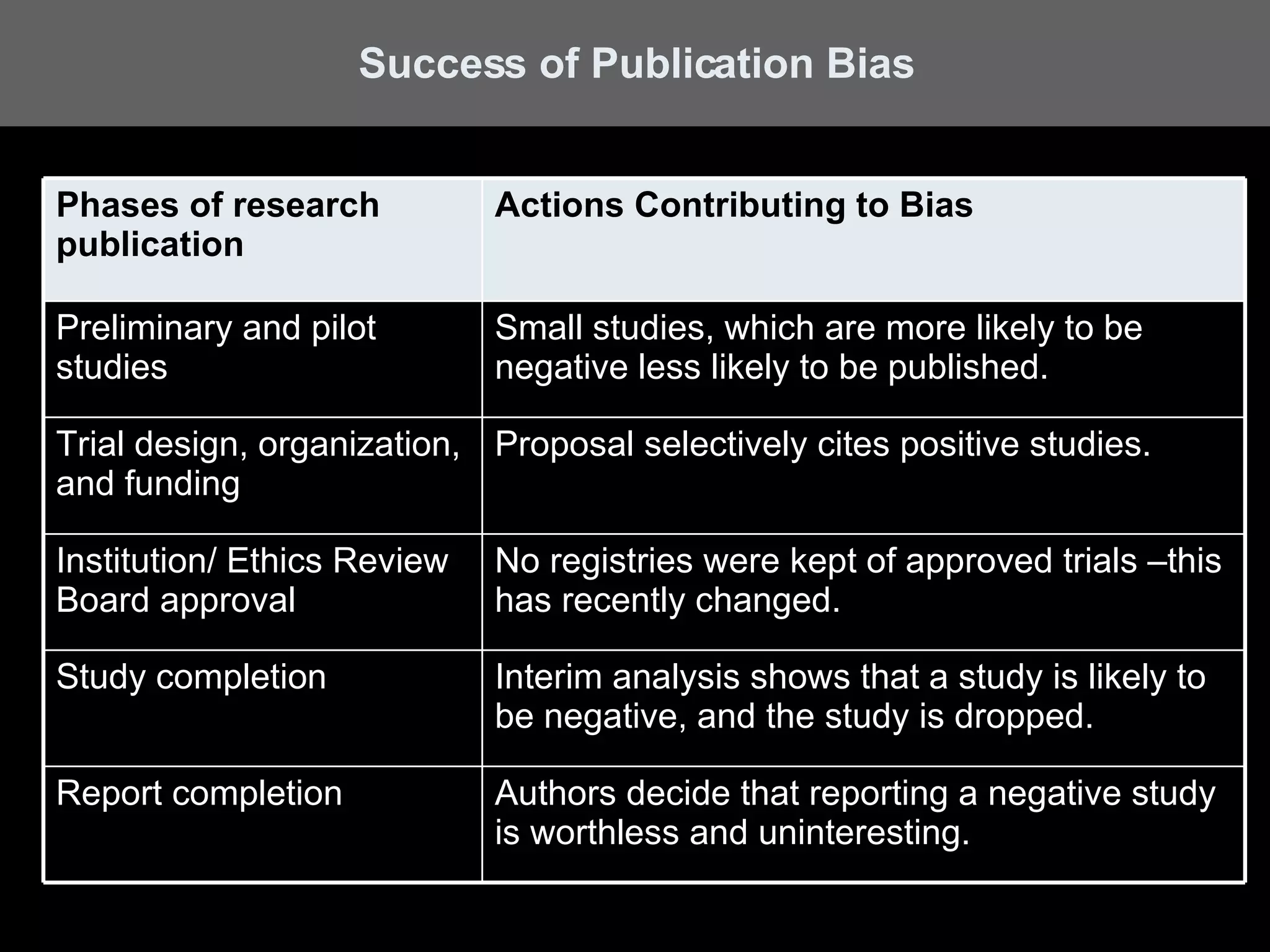
A systematic review is a literature review focused on answering a specific question by identifying, appraising, selecting, and synthesizing high-quality research evidence relevant to that question. It follows a rigorous methodology to overcome bias, including formulating a research question, conducting a comprehensive literature search, applying inclusion/exclusion criteria, assessing study quality, and analyzing results. The results are often combined using meta-analysis to provide a quantitative summary of effects across multiple studies.