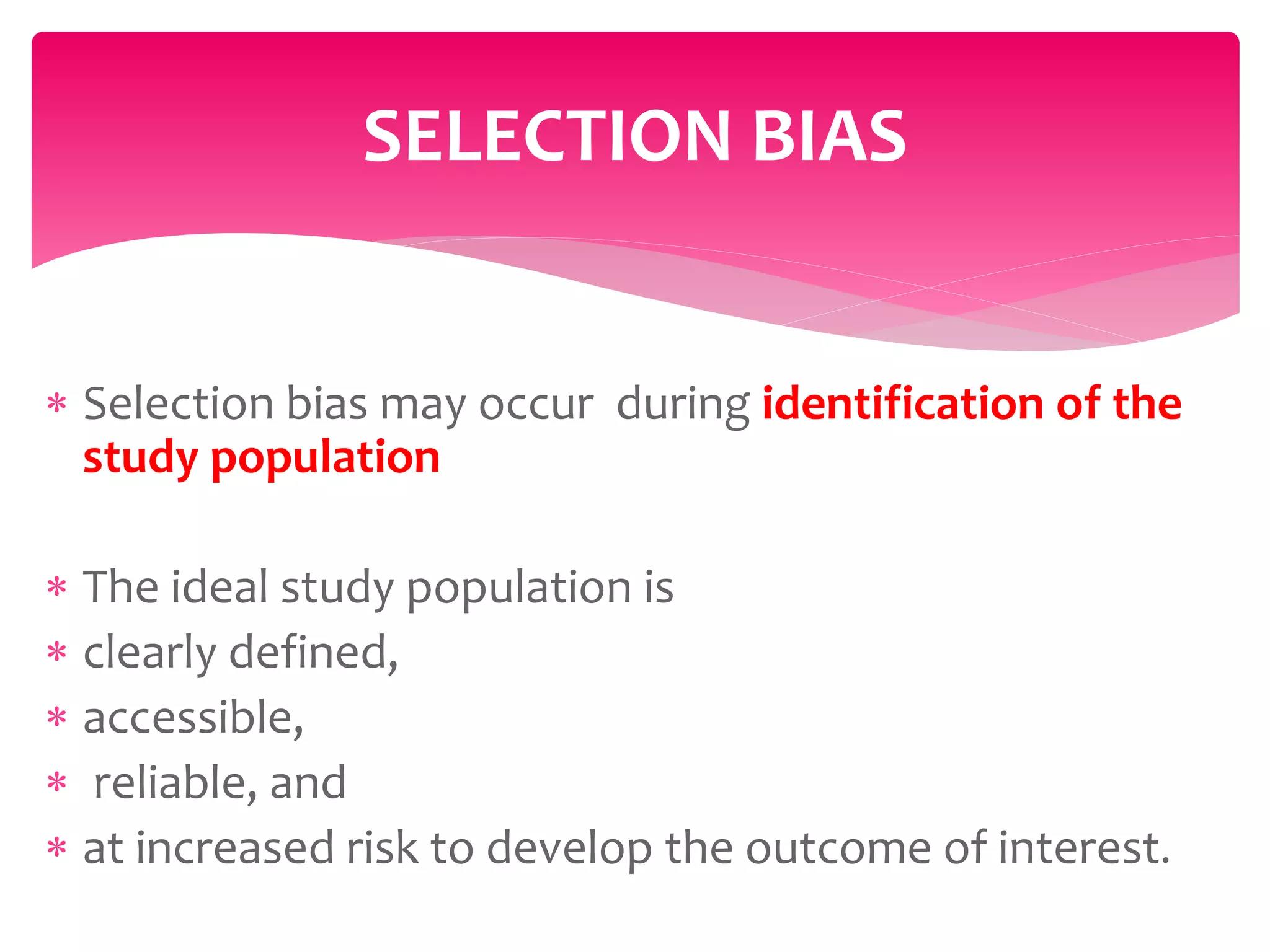
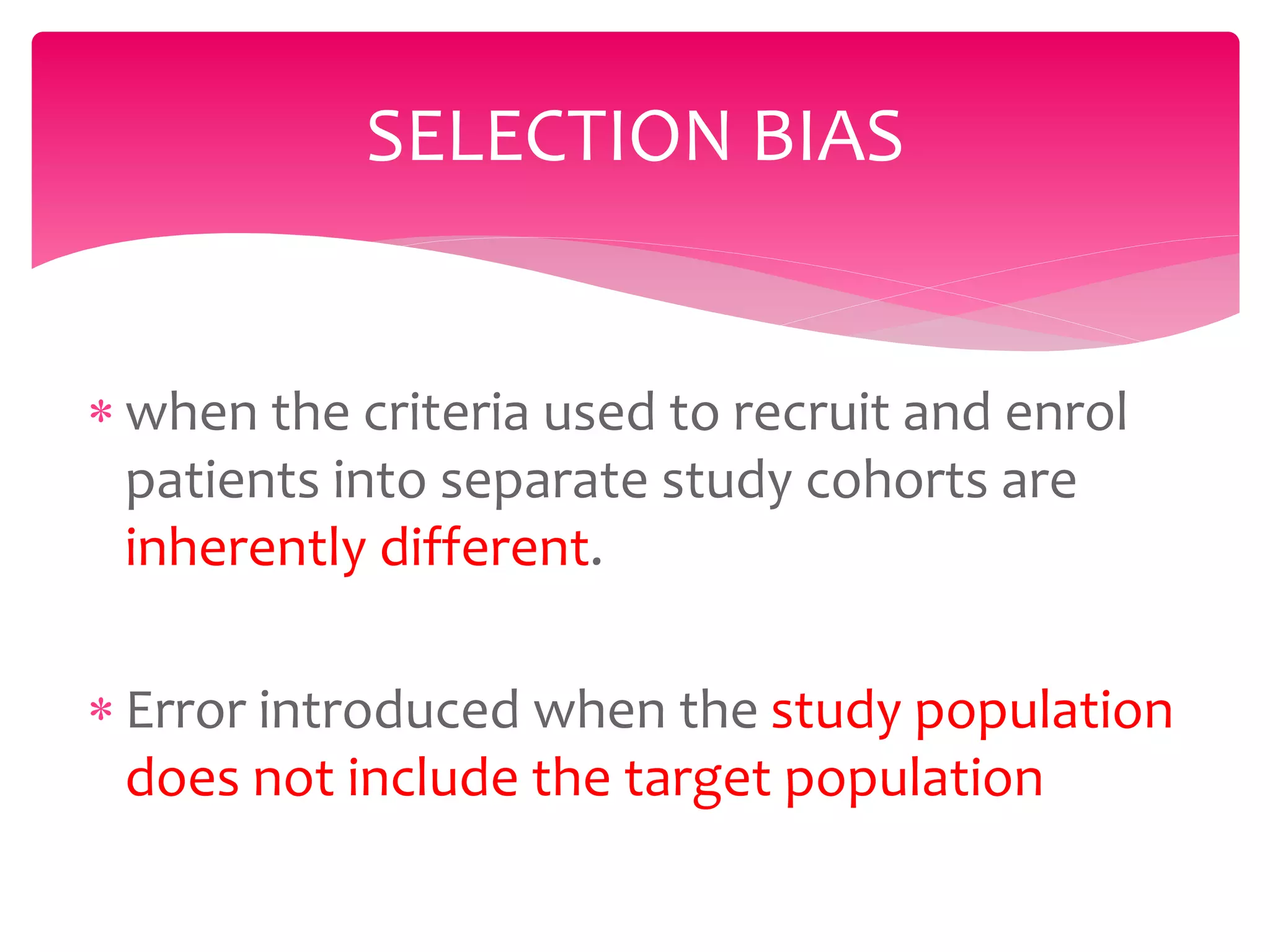
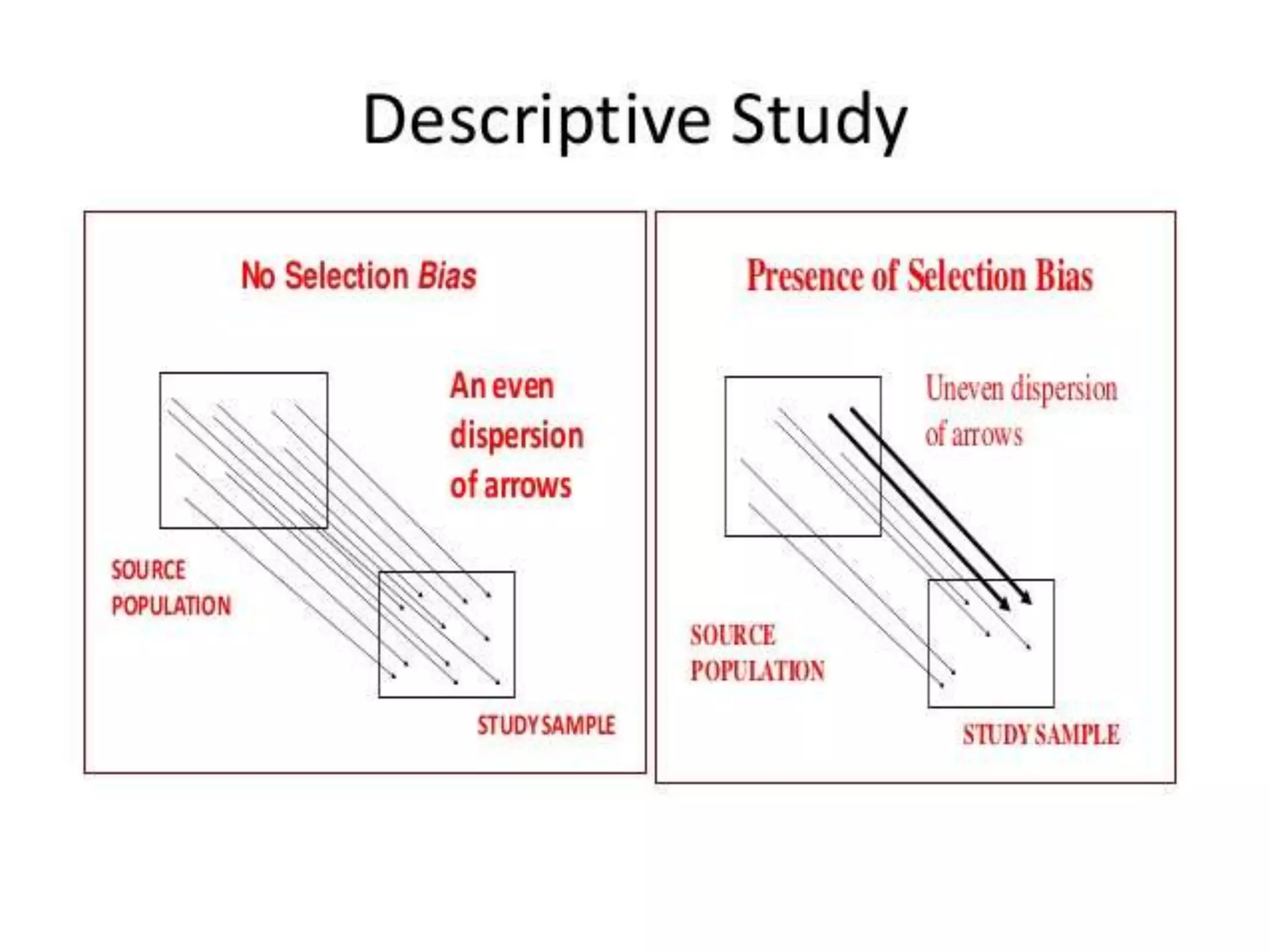
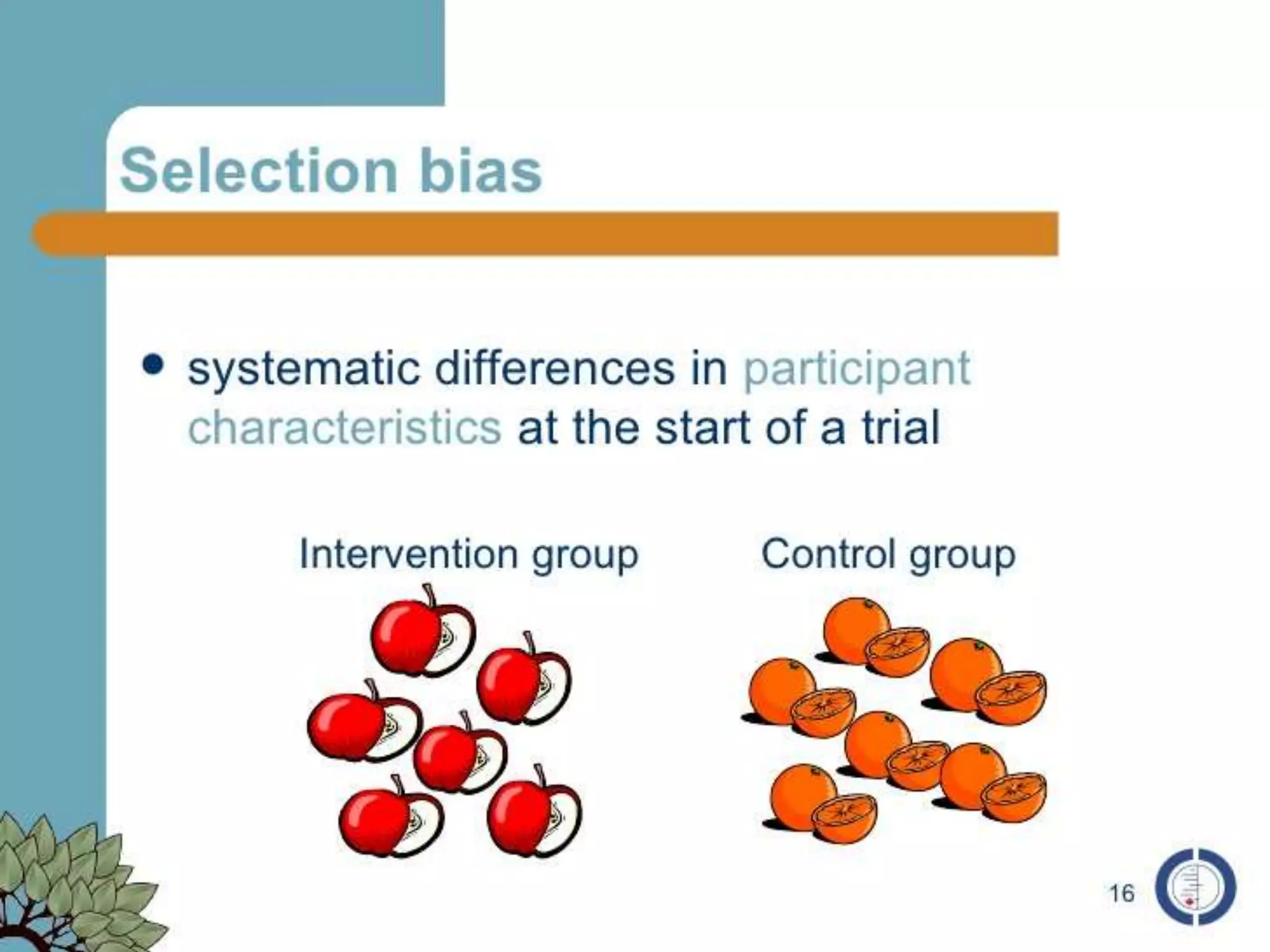
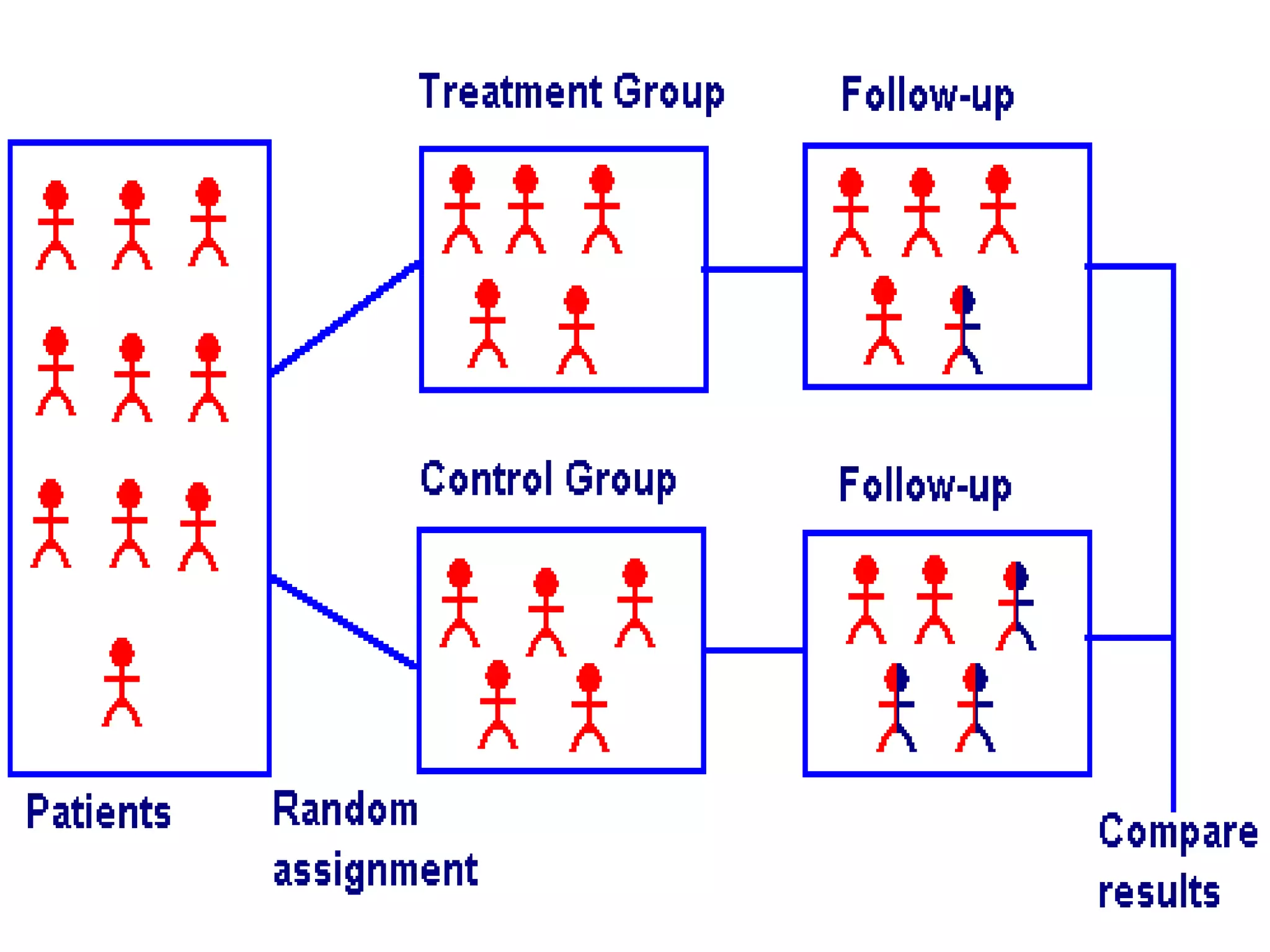
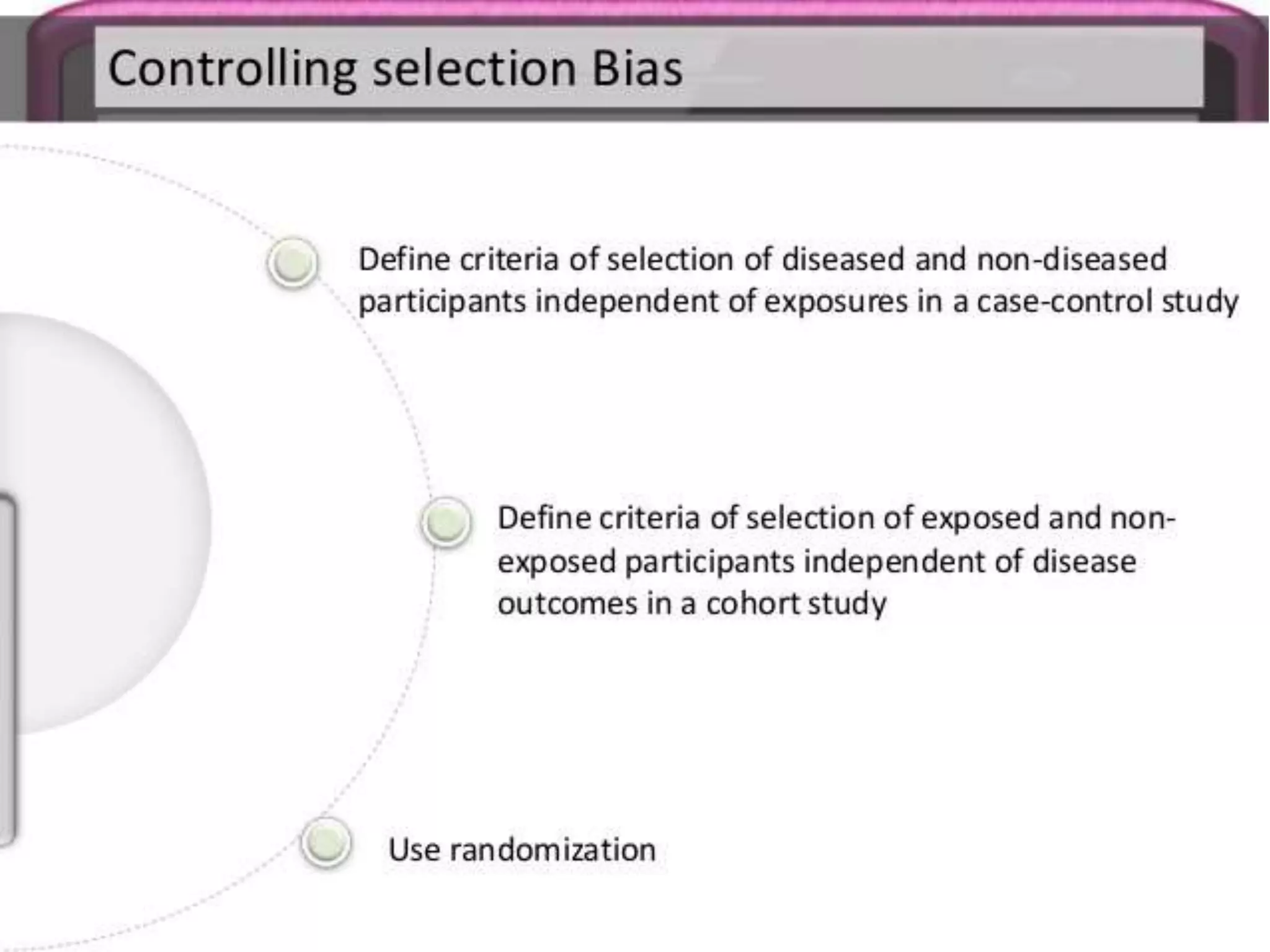
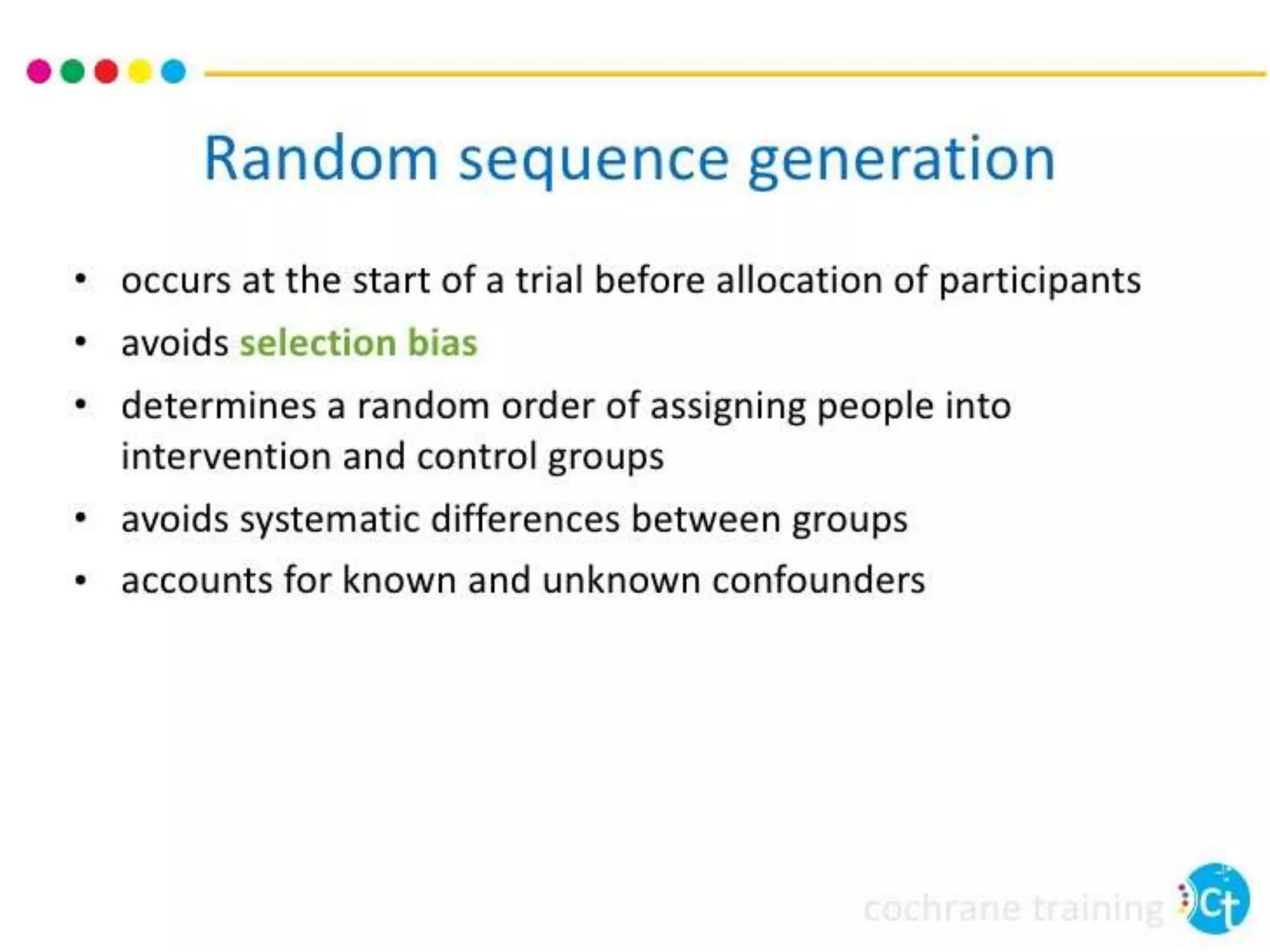
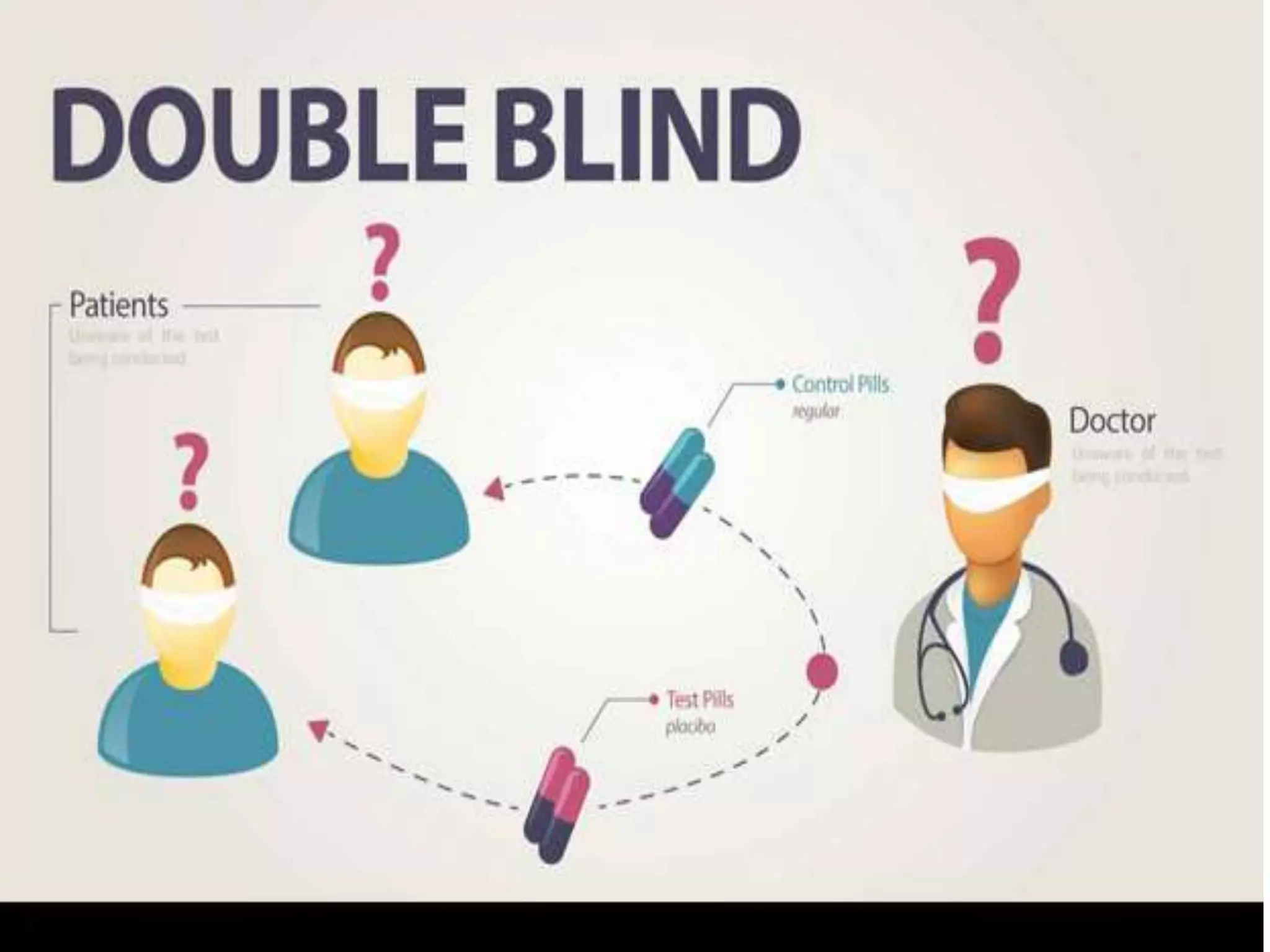
The document discusses various types of bias in clinical research, defining bias as a systematic deviation from truth that can result in invalid study outcomes. It outlines sources and classifications of bias, emphasizes methods to minimize them, and highlights the importance of internal and external validity in research. The document also provides case scenarios to illustrate potential biases and the significance of acknowledging limitations in research findings.







![systematic error [is] introduced into
sampling or testing by selecting or
encouraging one outcome or answer
over others
WHEN BIAS CAN HAPPEN ?](https://image.slidesharecdn.com/biasinclinicalresearch2017satyaslideshare-170922151651/75/Bias-in-clinical-research-8-2048.jpg)














































































![REFERENCES
1. 8.4 Introduction to sources of bias in clinical trials [Internet]. [cited 2014 Dec 22].
Available from:
http://handbook.cochrane.org/chapter_8/8_4_introduction_to_sources_of_bias_in_clinica
l_trials.htm
2. 9781405132664_4_003.indd - 9781405132664_4_003.pdf [Internet]. [cited
2014 Dec 22]. Available from:
http://www.blackwellpublishing.com/content/BPL_Images/Content_store/Sample_chapter/
9781405132664/9781405132664_4_003.pdf
3. Delgado-Rodríguez M, Llorca J. Bias. J Epidemiol Community Health. 2004 Aug
1;58(8):635–41.
4. bias.aspx [Internet]. [cited 2014 Dec 22]. Available from:
http://www.ashpfoundation.org/mainmenucategories/researchresourcecenter/fosteringyo
unginvestigators/ajhpresearchfundamentalsseries/bias.aspx
5. Sackett DL. Bias in analytic research. J Chronic Dis. 1979;32(1-2):51–63.](https://image.slidesharecdn.com/biasinclinicalresearch2017satyaslideshare-170922151651/75/Bias-in-clinical-research-99-2048.jpg)
![REFERENCES
6. Kopec JA, Esdaile JM. Bias in case-control studies. A review. J Epidemiol
Community Health. 1990 Sep;44(3):179–86.
7. FEM - Preventing bias [Internet]. [cited 2014 Dec 22]. Available from:
https://wiki.ecdc.europa.eu/fem/w/fem/preventing-bias.aspx
8. Pannucci CJ, Wilkins EG. Identifying and Avoiding Bias in Research. Plast
Reconstr Surg. 2010 Aug;126(2):619–25.
9. Qualitative Research Bias - How to Recognize It [Internet]. [cited 2014 Dec
22]. Available from: http://www.focusgrouptips.com/qualitative-research.html
10. Types of bias.pdf [Internet]. [cited 2014 Dec 22]. Available from:
http://www.medicalbiostatistics.com/Types%20of%20bias.pdf](https://image.slidesharecdn.com/biasinclinicalresearch2017satyaslideshare-170922151651/75/Bias-in-clinical-research-100-2048.jpg)
