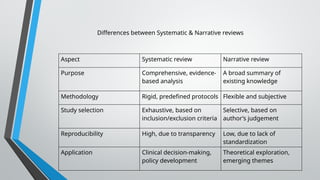
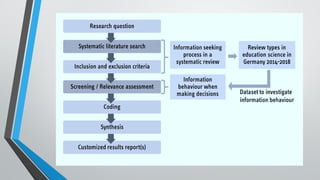
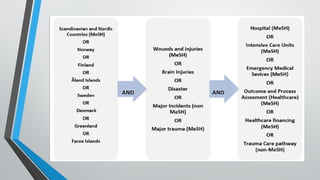
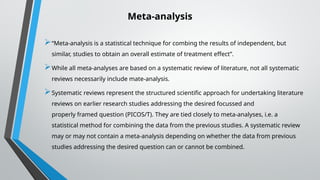
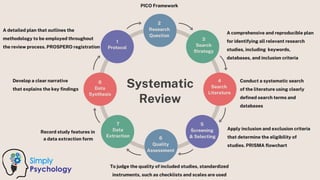
A systematic review and meta-analysis are essential tools in evidence-based research. It covers formulating research questions (PICO), literature search strategies, study selection, data extraction, quality assessment (e.g., PRISMA, Cochrane ROB), and statistical techniques for meta-analysis (e.g., forest plots, heterogeneity tests). The slides also address interpretation, reporting guidelines, and practical applications, making it a valuable resource for researchers, clinicians, and students aiming to synthesize high-quality evidence.