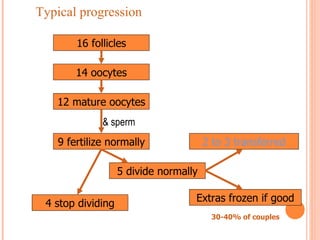
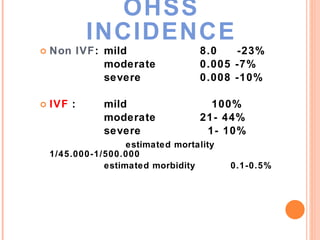
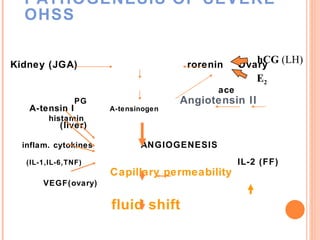
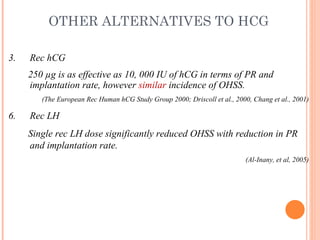
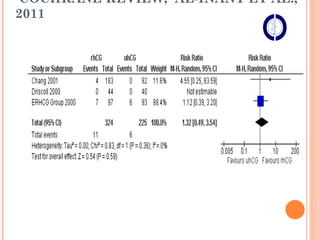
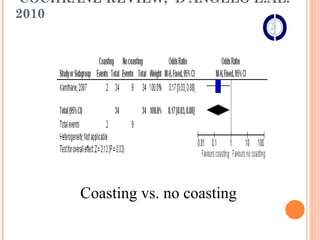
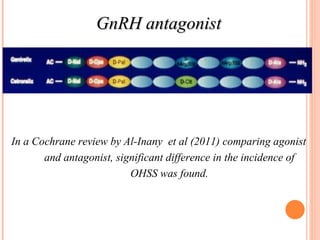
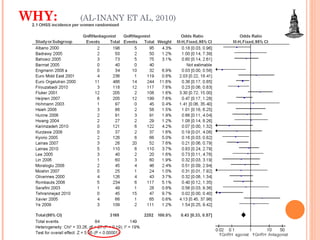
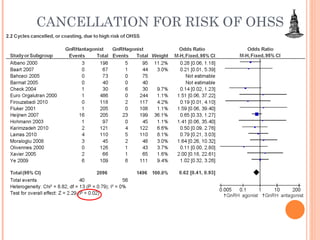
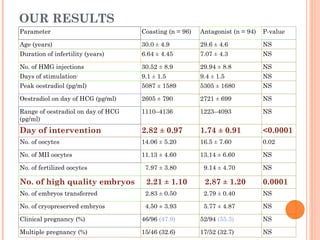
This document discusses strategies for preventing severe ovarian hyperstimulation syndrome (OHSS) during in vitro fertilization treatment. It describes the pathogenesis of OHSS and risk factors. Methods for prevention discussed include using a mild stimulation protocol, metformin treatment, coasting by withholding gonadotropins before triggering ovulation, using a GnRH antagonist protocol instead of agonist, cryopreserving all embryos, and administering intravenous albumin or hydroxyethyl starch after oocyte retrieval to reduce risk. The document concludes that OHSS is a preventable complication through various medical interventions and protocols.