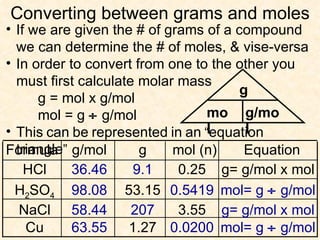
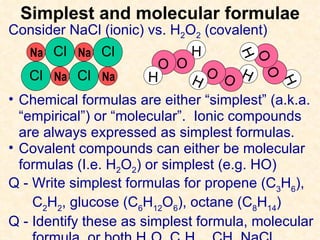
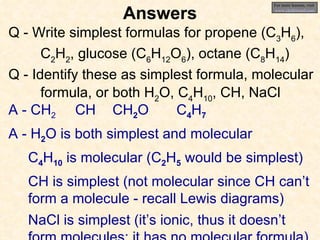
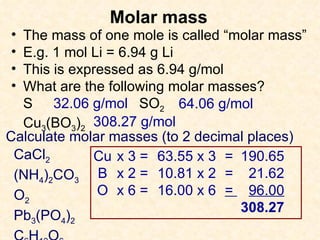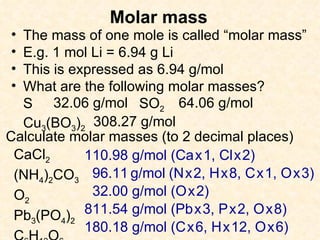The document discusses several chemistry concepts including:
- It would take 19 million years to spend a mole of $1 coins (6.02 x 10^23 coins) if spent at a rate of 1 billion per second.
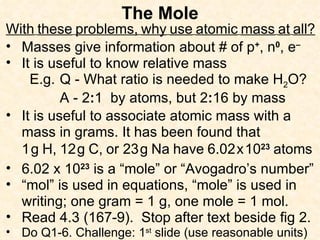
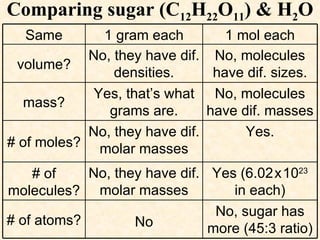
- Atomic masses on the periodic table represent relative masses of atoms and are useful for determining ratios and masses in grams of elements and compounds.
- A mole is a number (6.02 x 10^23) that allows easy conversion between atomic/molar masses and numbers of atoms/molecules.