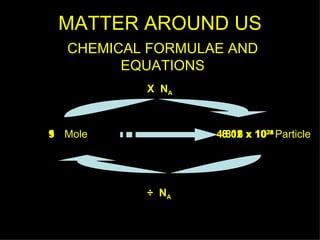
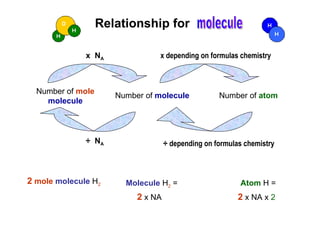
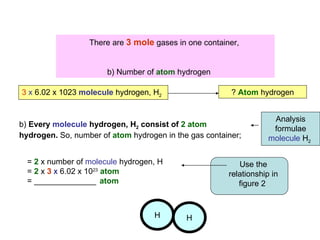
1) The document discusses the relationships between the number of particles, molecules, ions, and atoms that make up different chemical substances using moles as the unit of measurement.
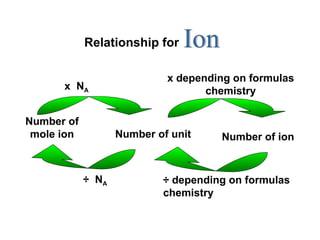
2) Key relationships shown include that the number of particles in 1 mole of a substance is equal to Avogadro's constant, and the number of molecules, ions or atoms can be calculated by multiplying the number of moles by Avogadro's constant and taking into account the chemical formula.
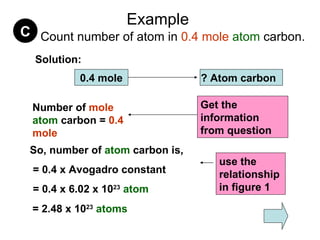
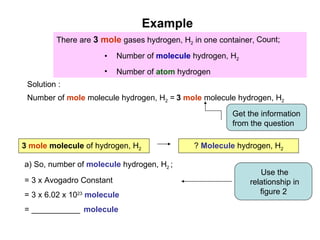
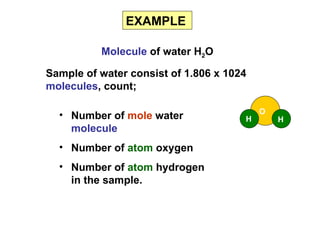
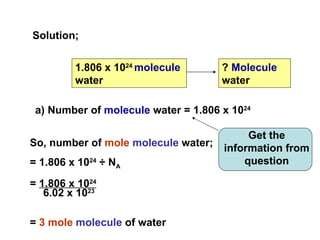
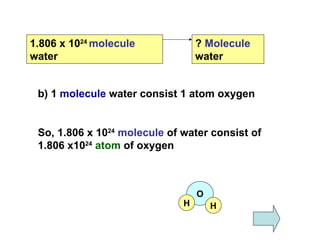
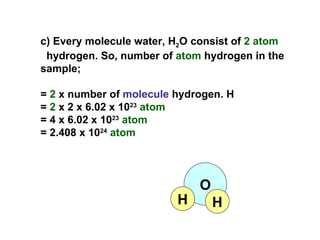
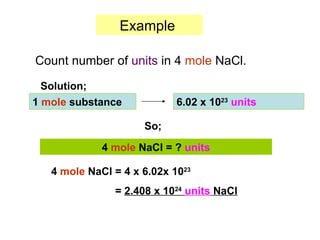
3) Examples are provided to demonstrate calculating the number of molecules, ions and atoms given the number of moles of different substances like carbon, hydrogen gas, water and sodium chloride.