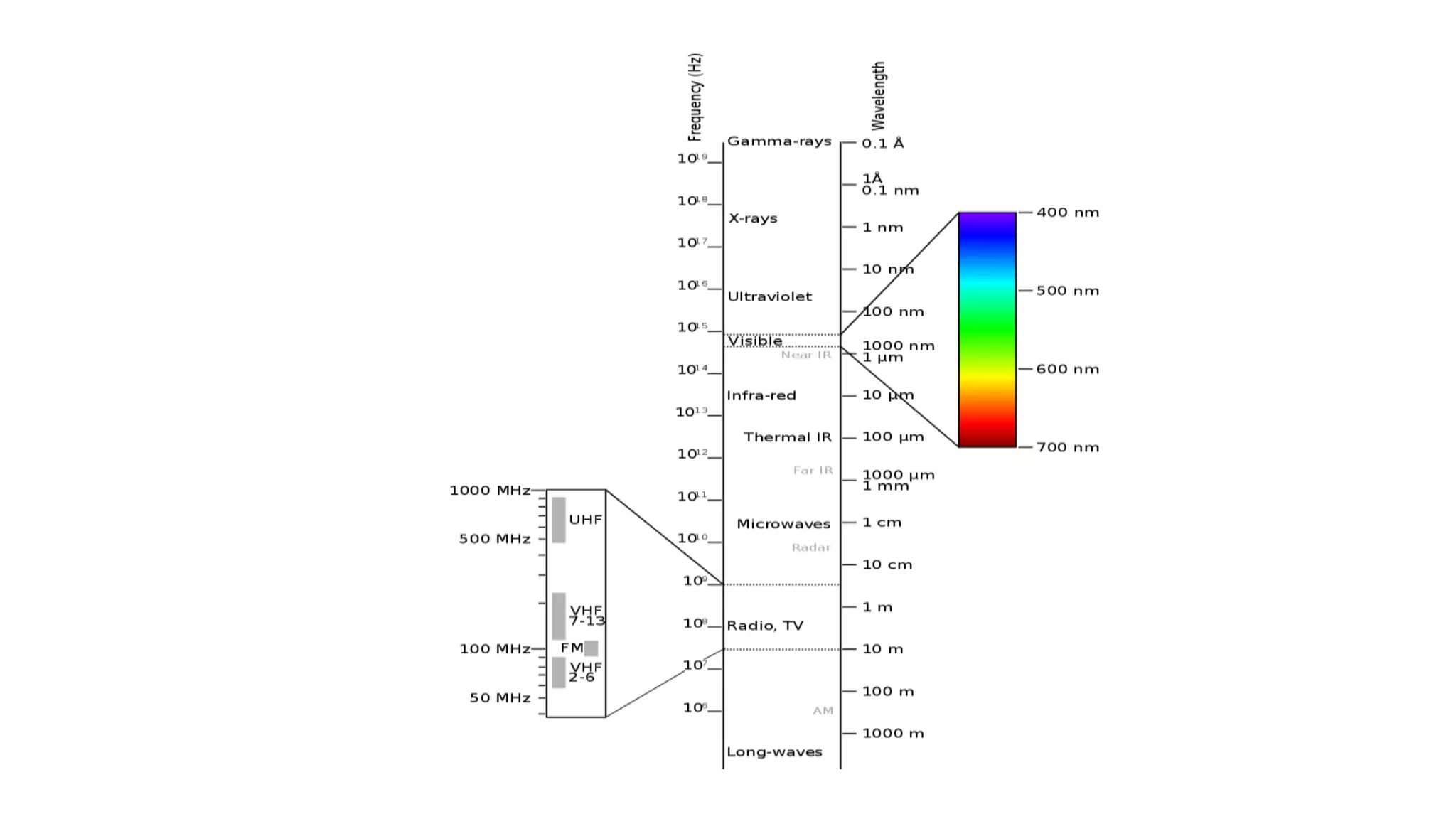
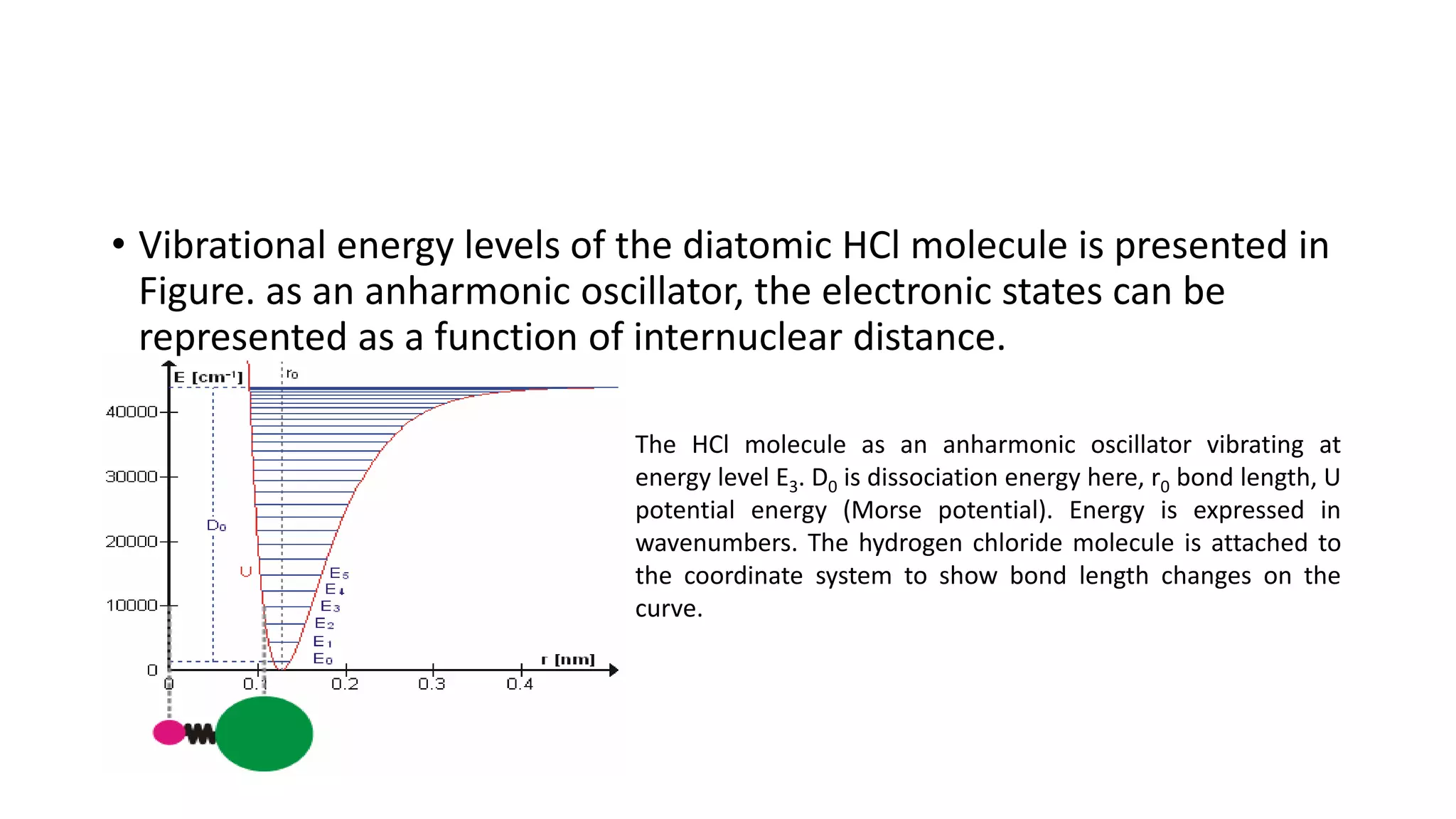
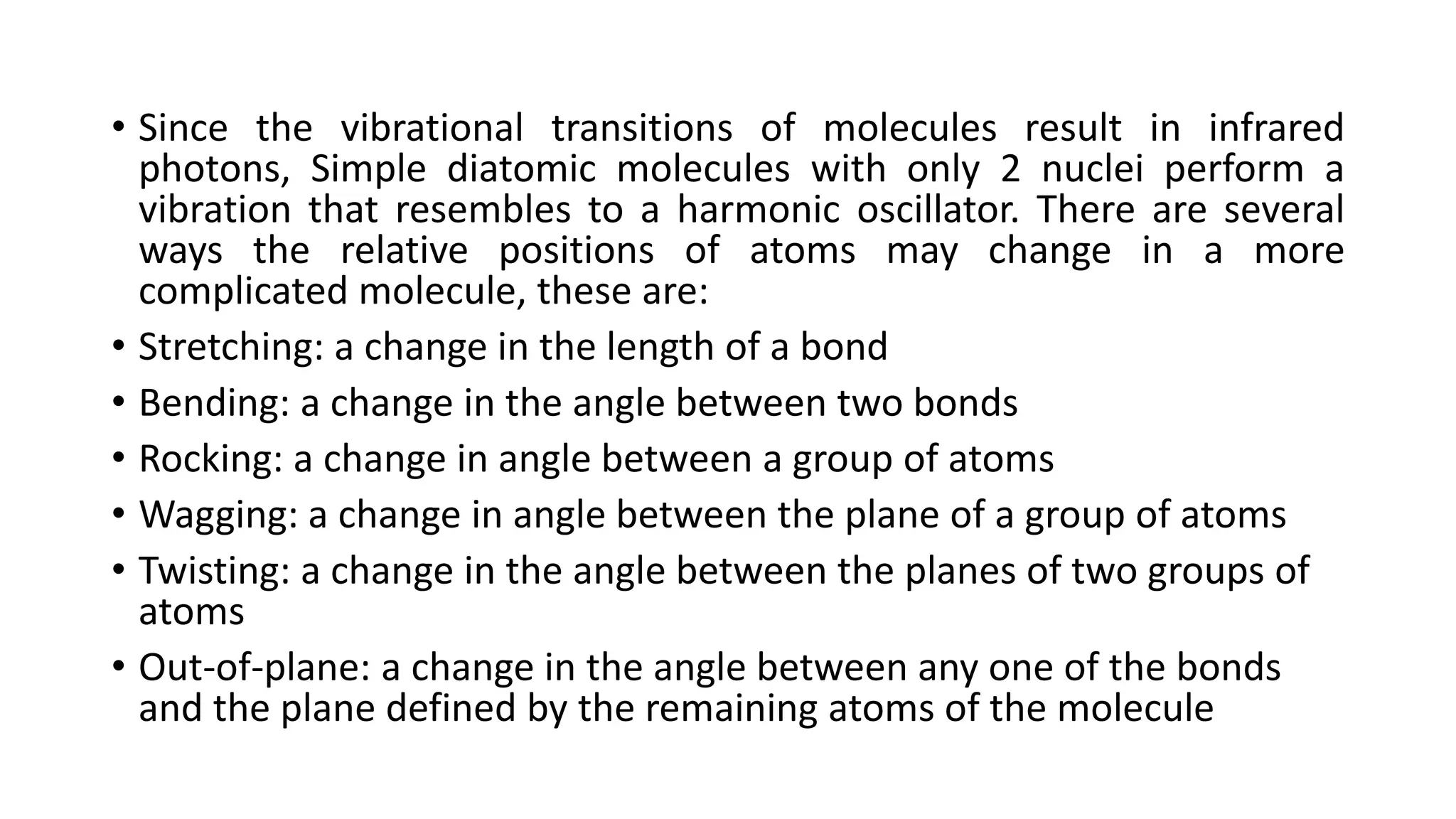
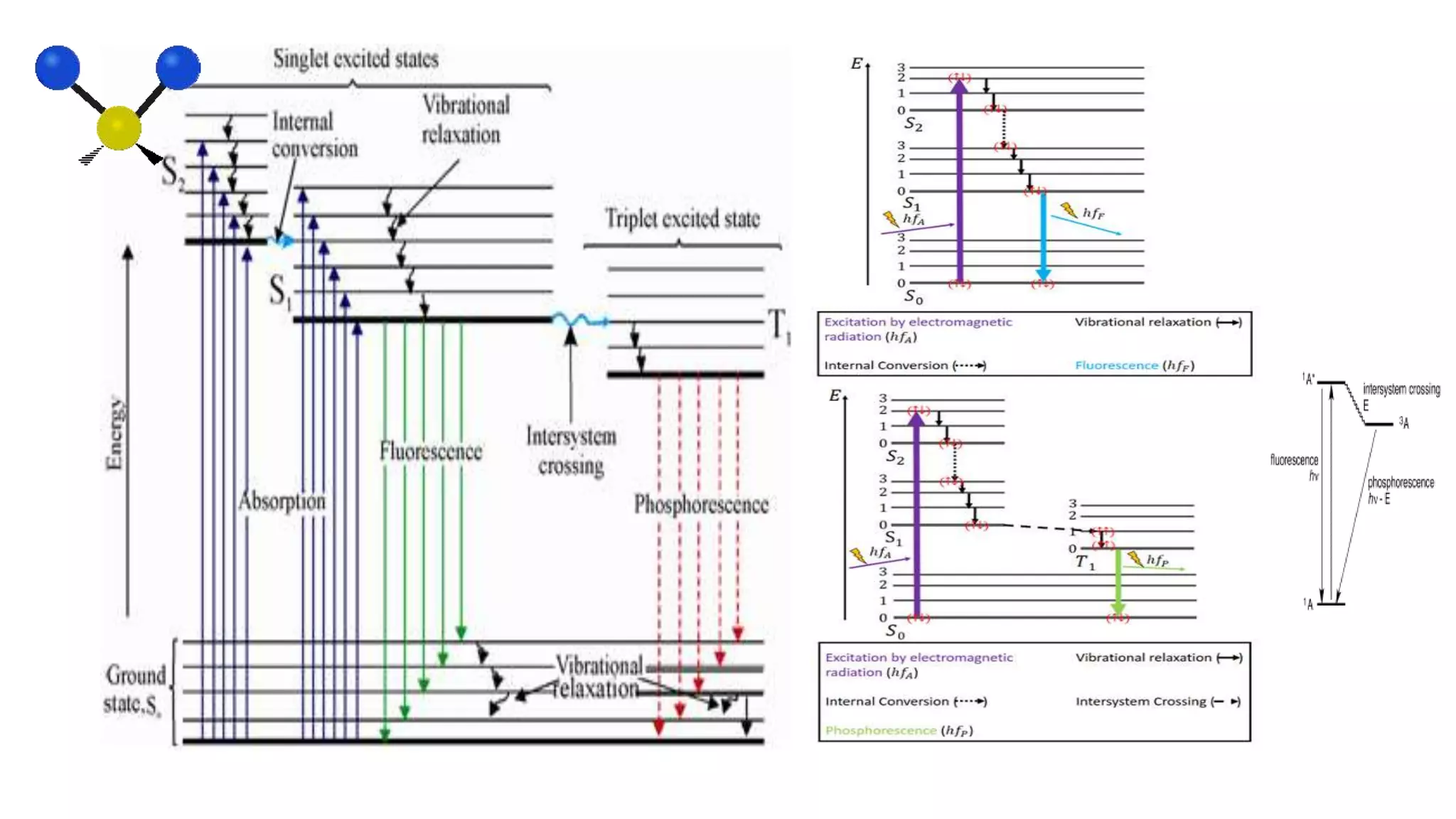
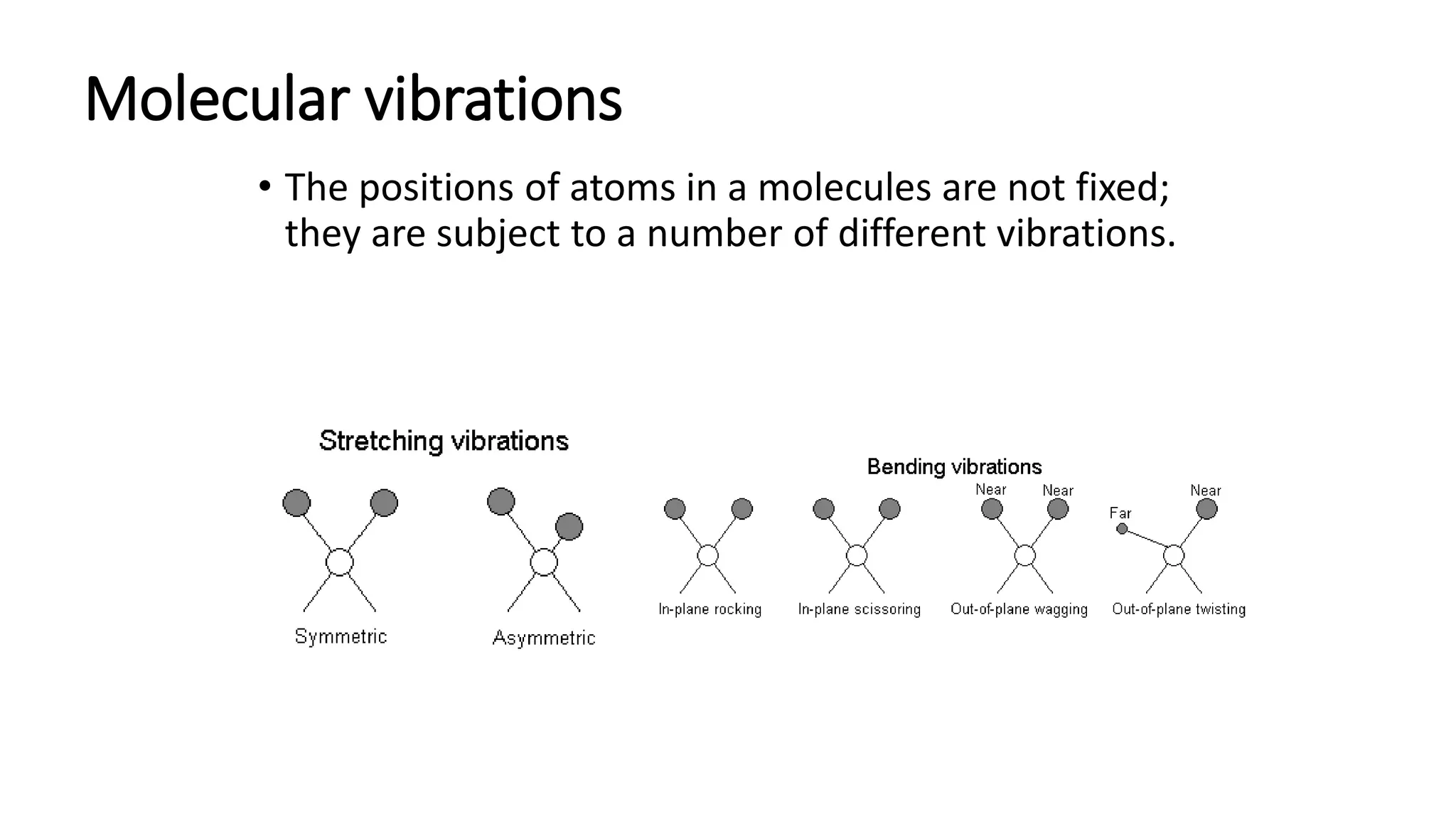
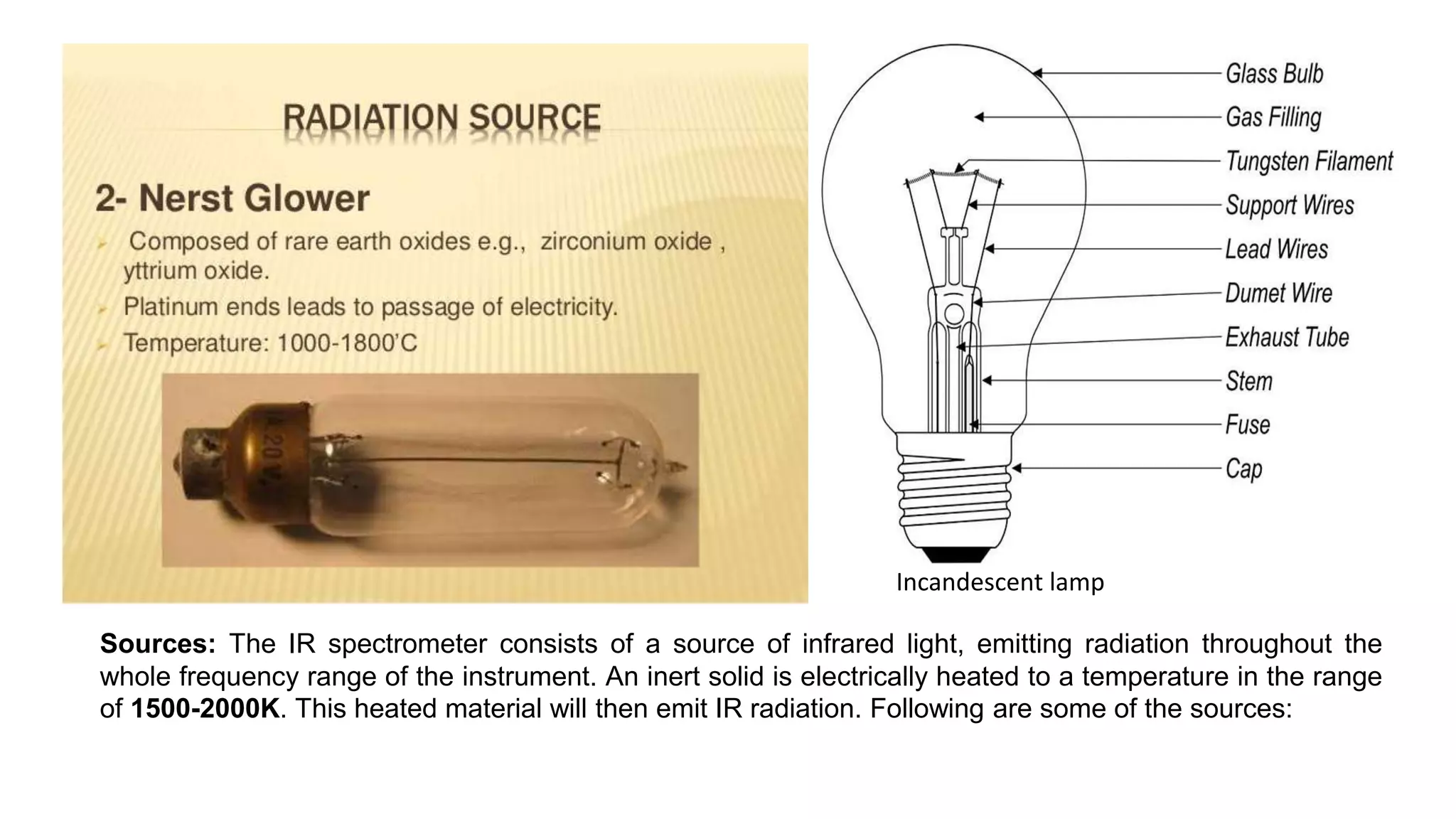
This document provides an overview of infrared (IR) spectroscopy and IR spectrophotometers. It discusses how IR spectroscopy works by detecting the absorption of IR radiation by molecules as they undergo transitions between vibrational and rotational energy levels. The key components of an IR spectrophotometer are described, including the IR radiation source, sample cells, monochromators to select wavelengths, detectors to measure absorption, and recorders to display the spectra. Common molecular vibrations that can be observed in IR spectra are also outlined.