Embed presentation
Download to read offline










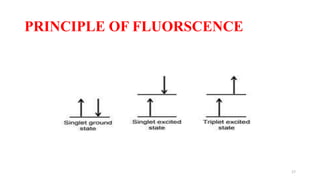
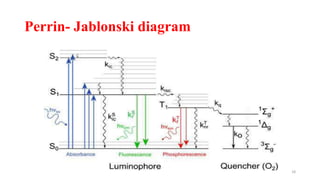
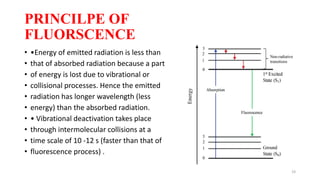

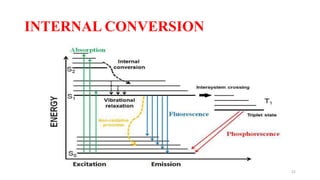
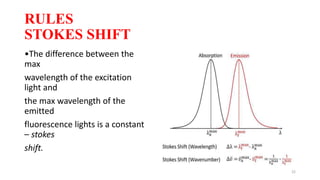
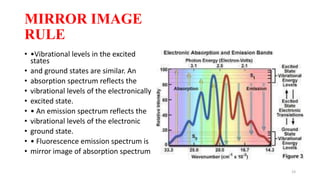
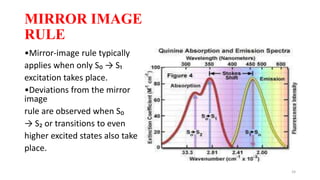
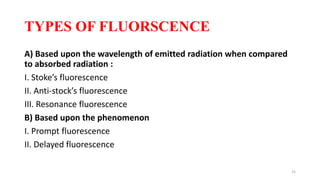

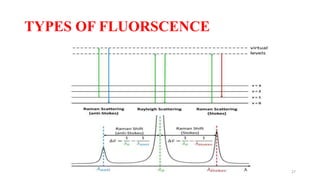









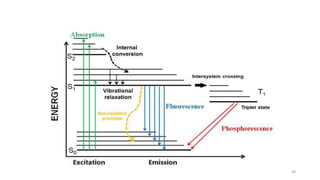

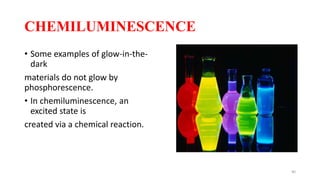

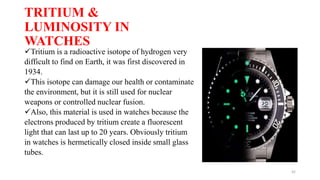



The document discusses bioluminescence, a phenomenon where living organisms produce light through chemical reactions, and it is prevalent in marine environments as well as in some terrestrial species. It also addresses fluorescence and phosphorescence, explaining their mechanisms, applications in various fields, and differences between them. Additionally, it highlights the historical background and discoveries related to these light-emitting processes.












































