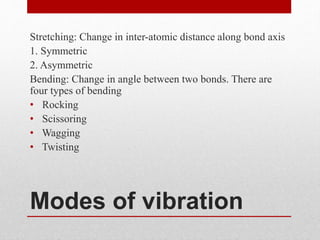
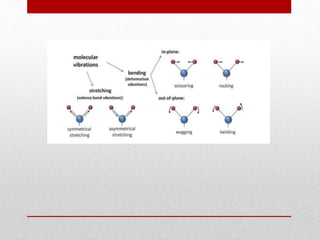
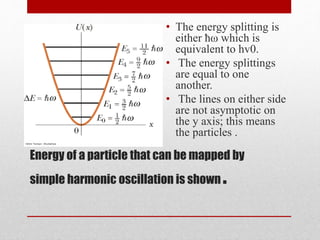
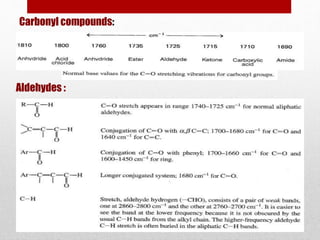
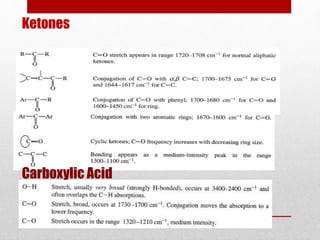
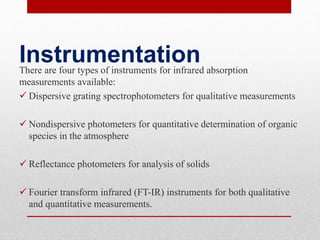
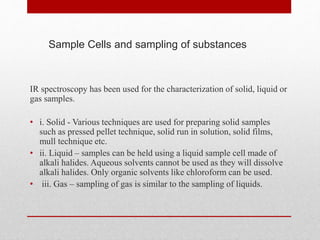
The document provides an overview of infrared (IR) spectroscopy, discussing its theory, modes of vibration, and various instrumentation used for measurements. It explains the electromagnetic spectrum range of IR, the significance of Hooke’s law in molecular vibrations, and details different types of IR spectrometers and their components. Additionally, it covers sample preparation techniques for solids, liquids, and gases, as well as the classification of detectors used in IR spectroscopy.