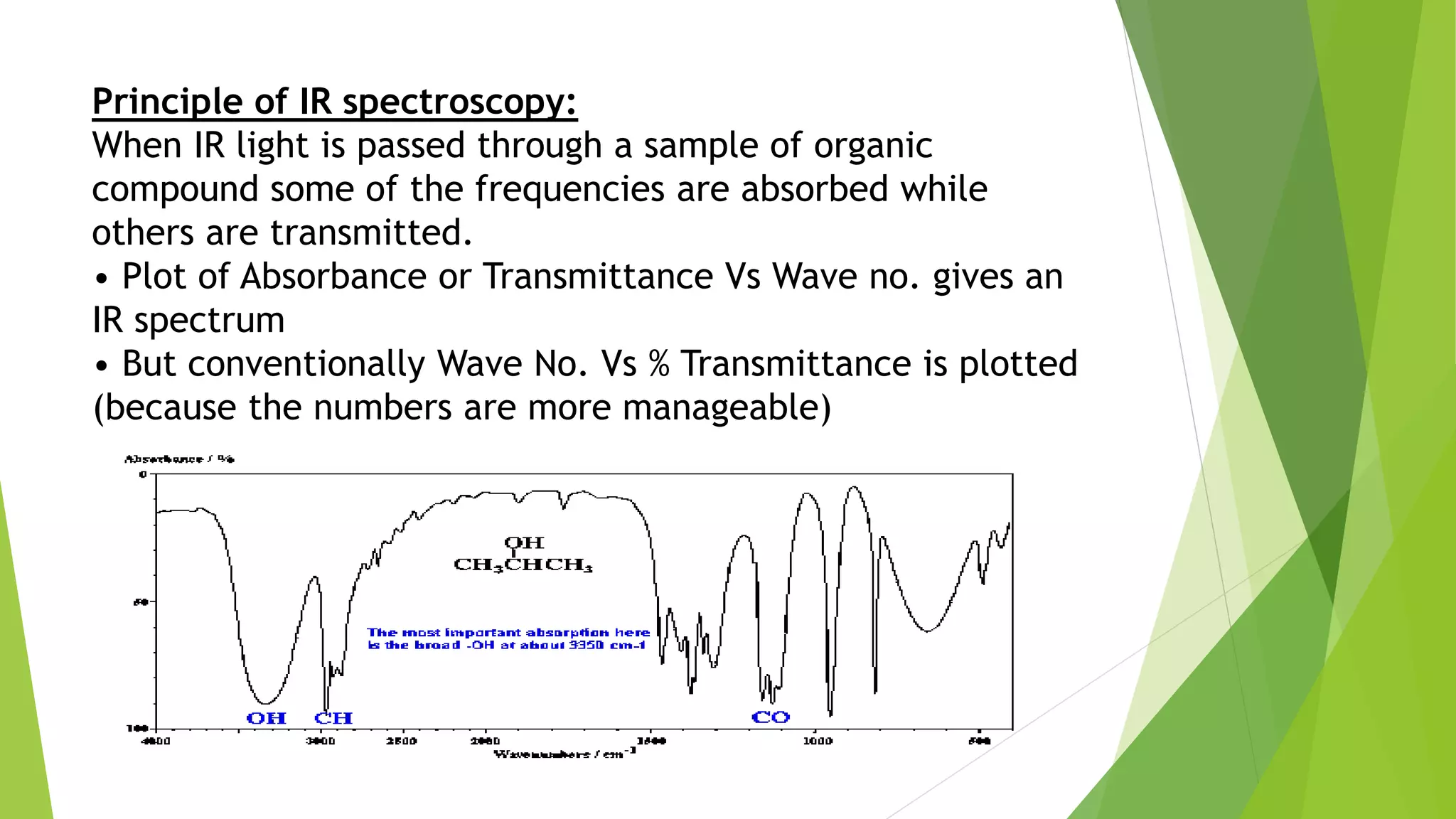
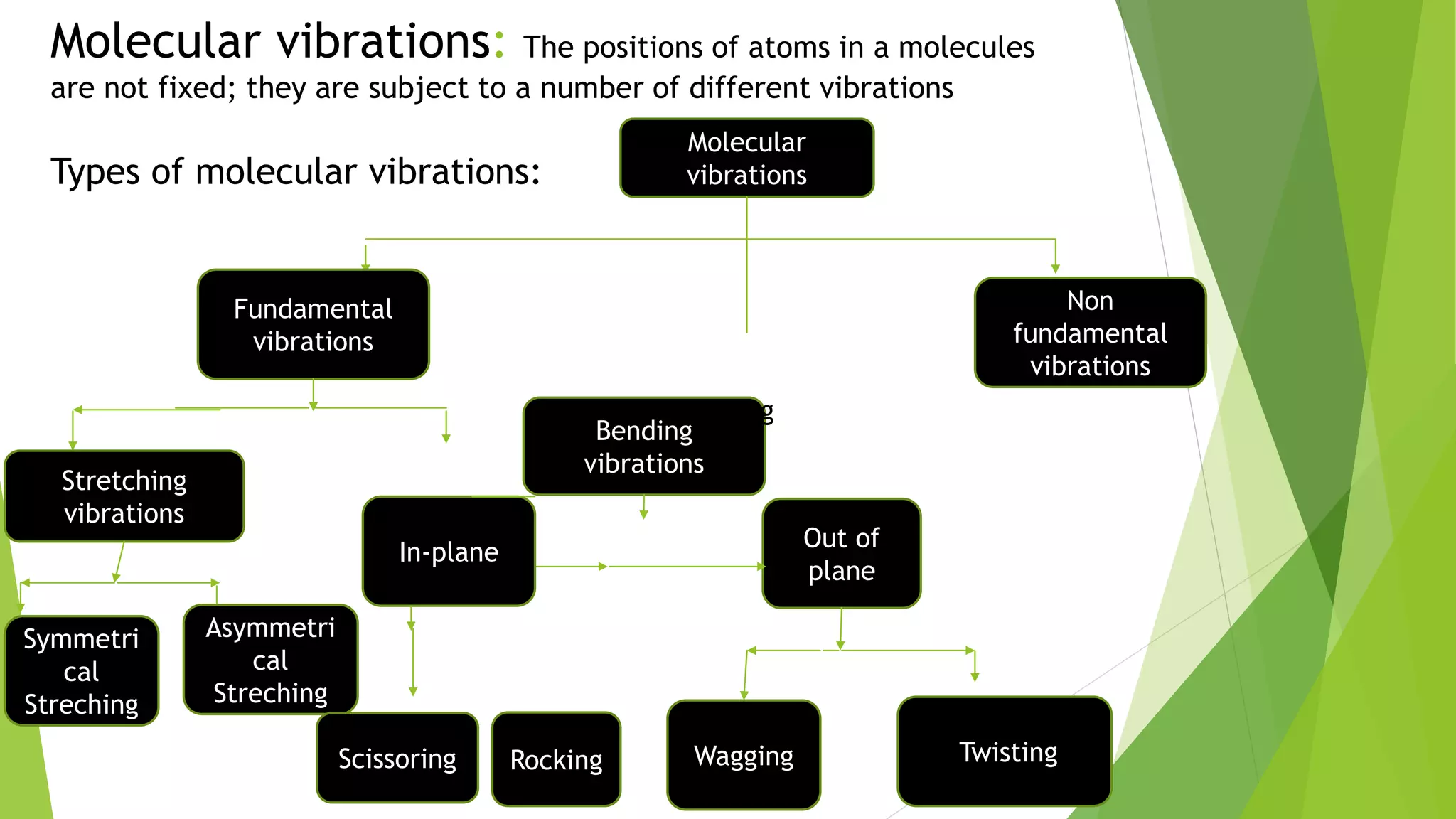
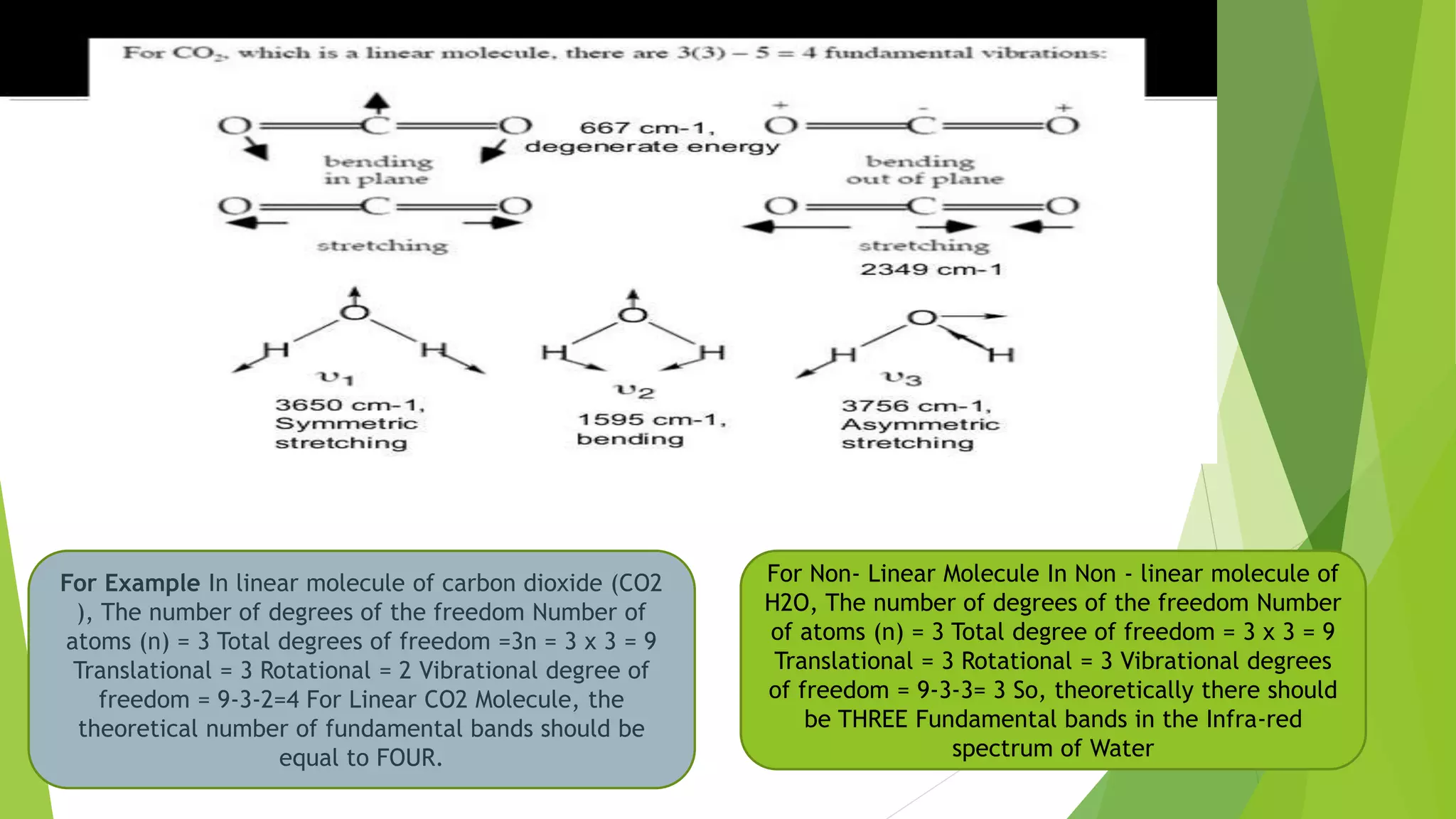
Spectroscopic techniques involve measuring the interaction of electromagnetic radiation with matter. There are various types of spectroscopy depending on the type of radiation used. Infrared (IR) spectroscopy analyzes infrared light interacting with molecules and is based on absorption spectroscopy. IR spectroscopy is useful for qualitative and quantitative analysis, detecting impurities, and characterizing organic compounds. Molecular vibrations that can be analyzed include stretching vibrations, which change bond lengths, and bending vibrations, which change bond angles. Selection rules determine which vibrations are IR active based on whether they induce a change in the molecule's dipole moment.