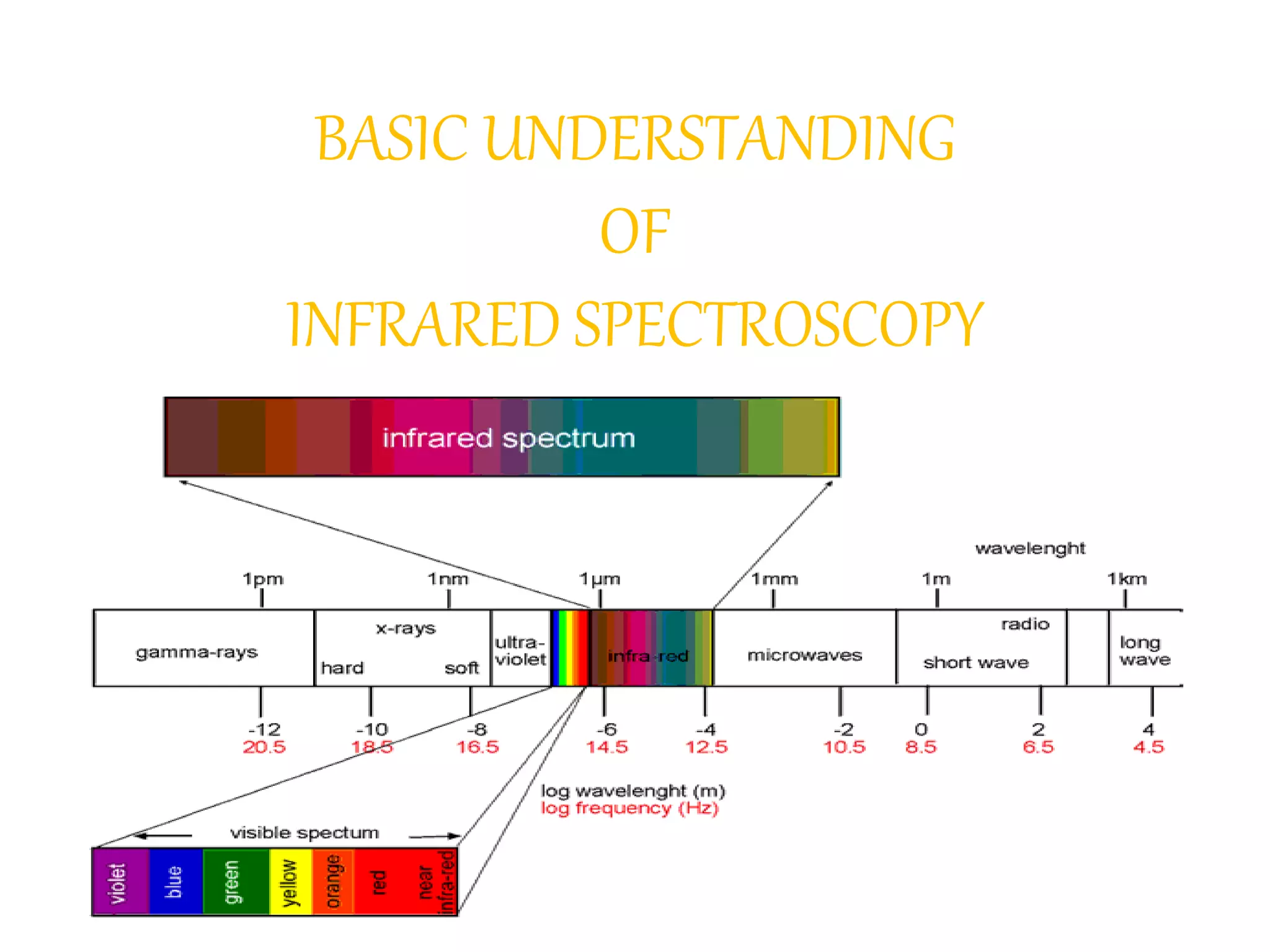
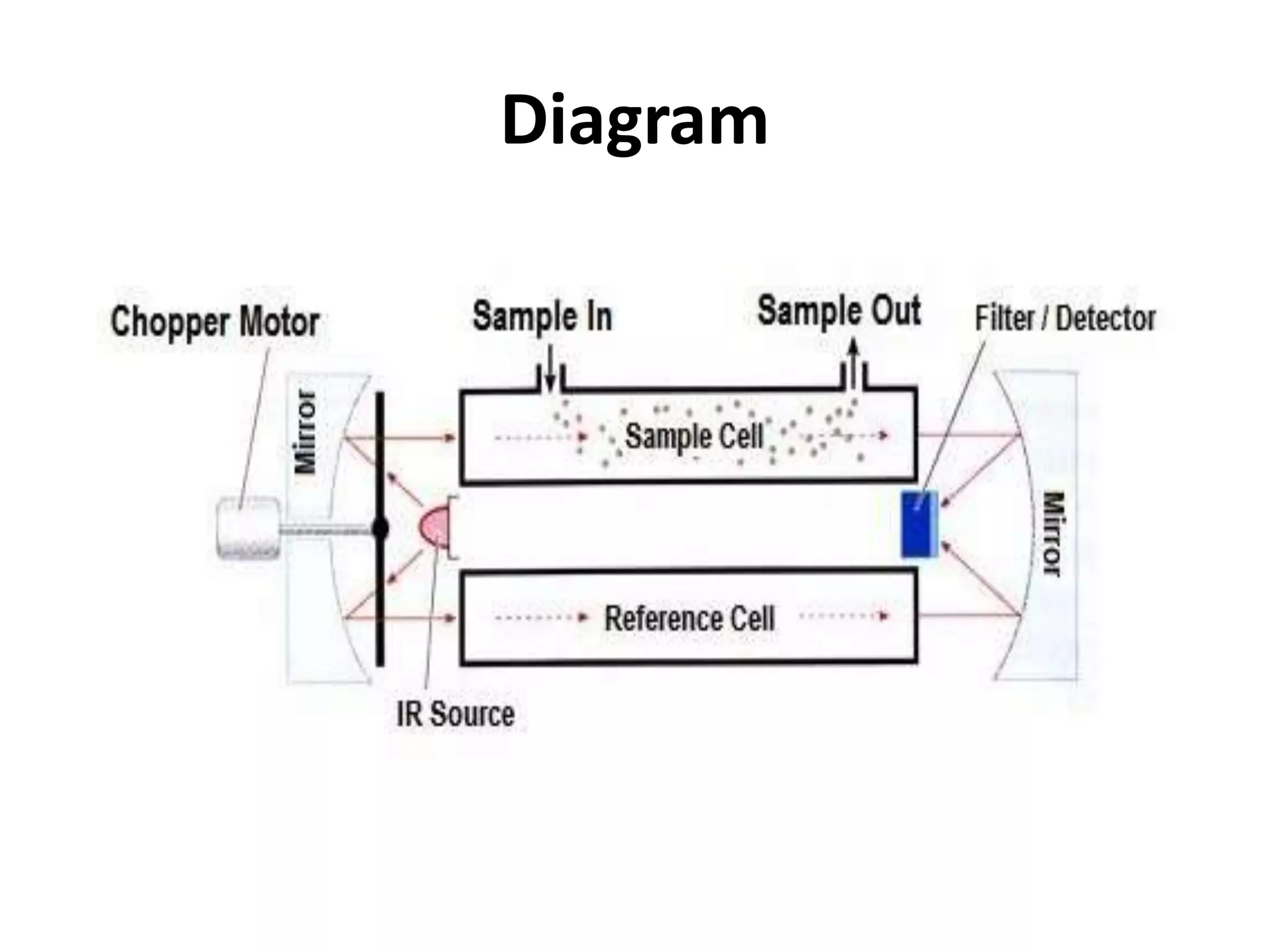
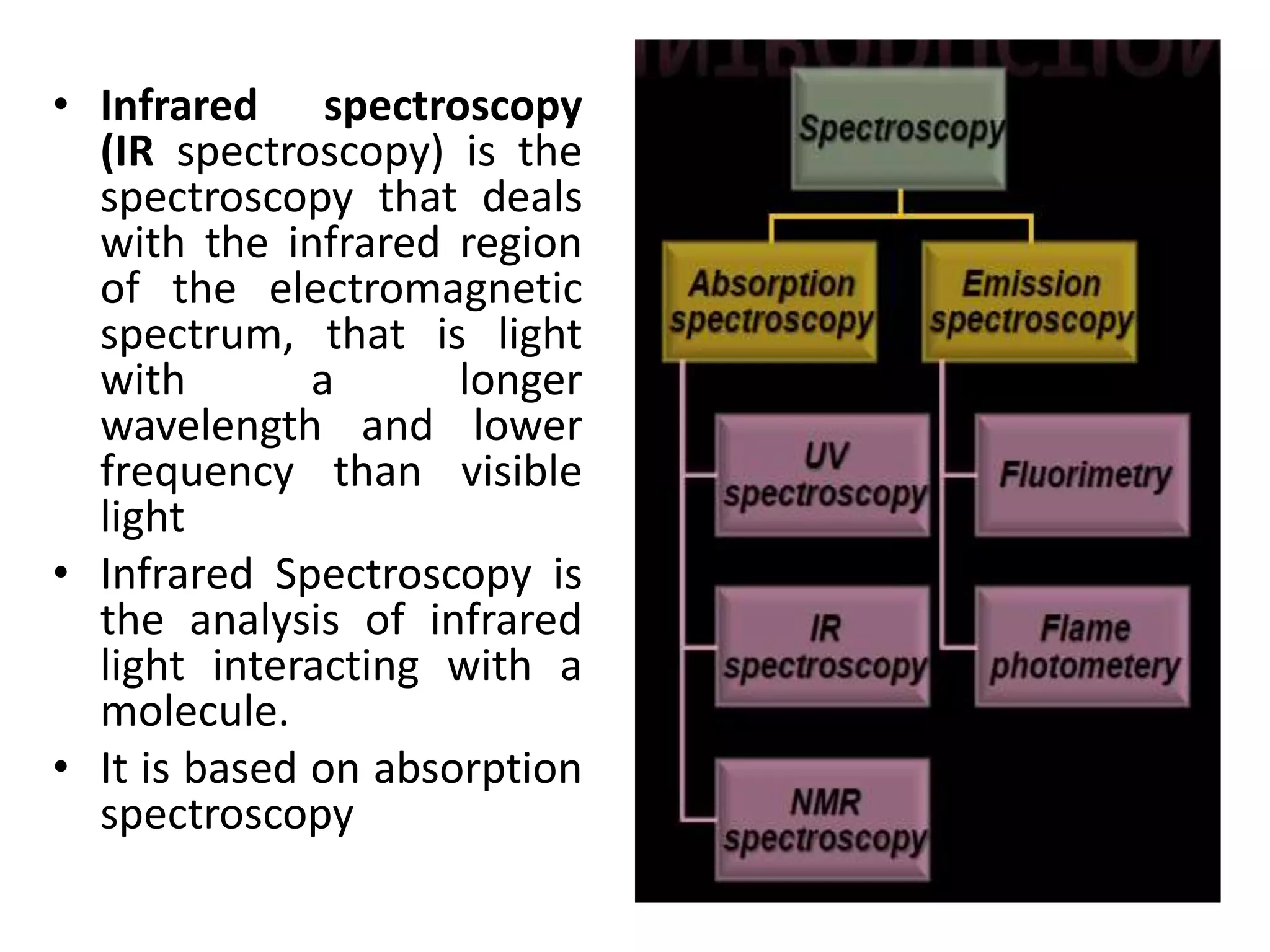
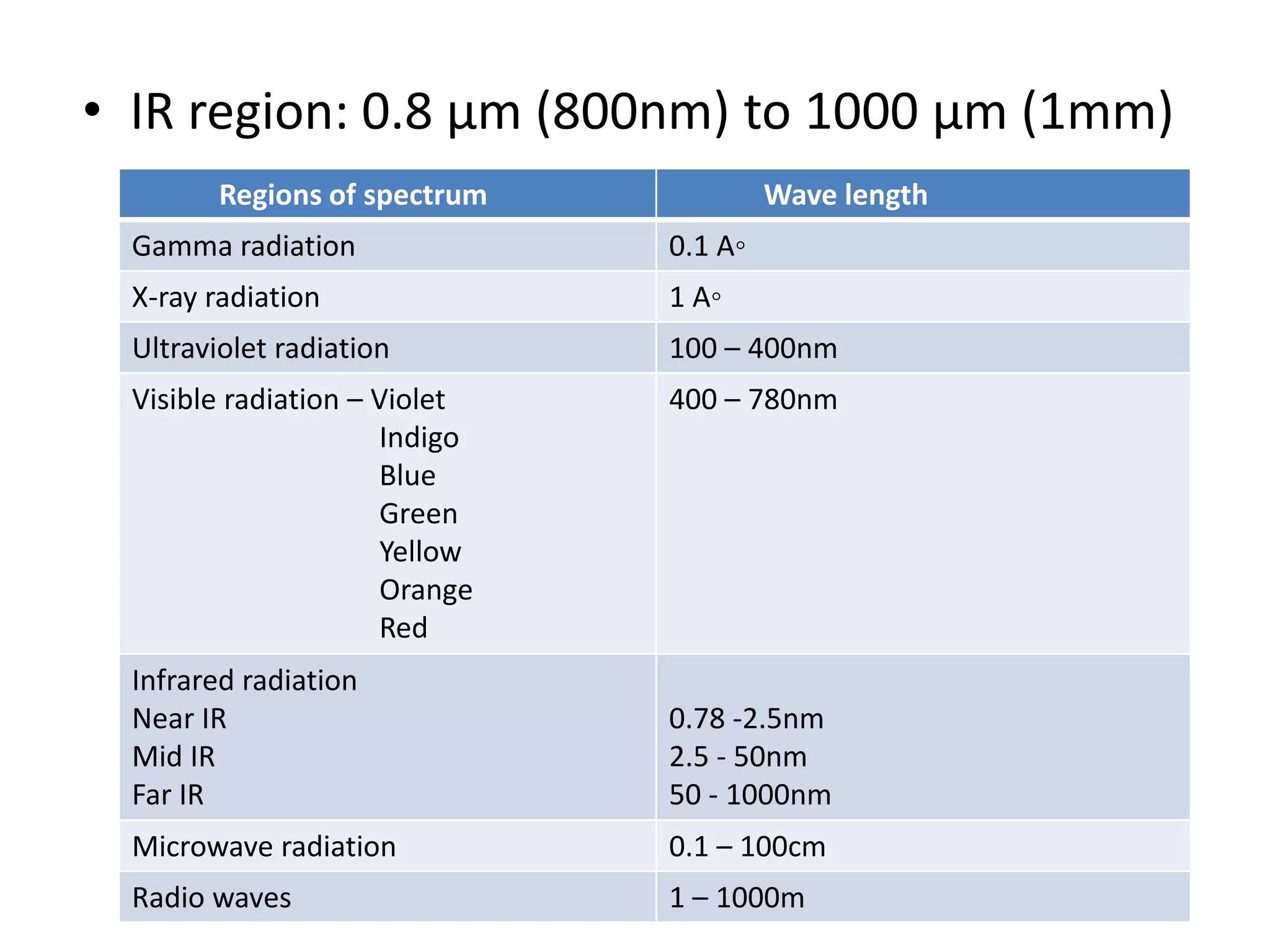
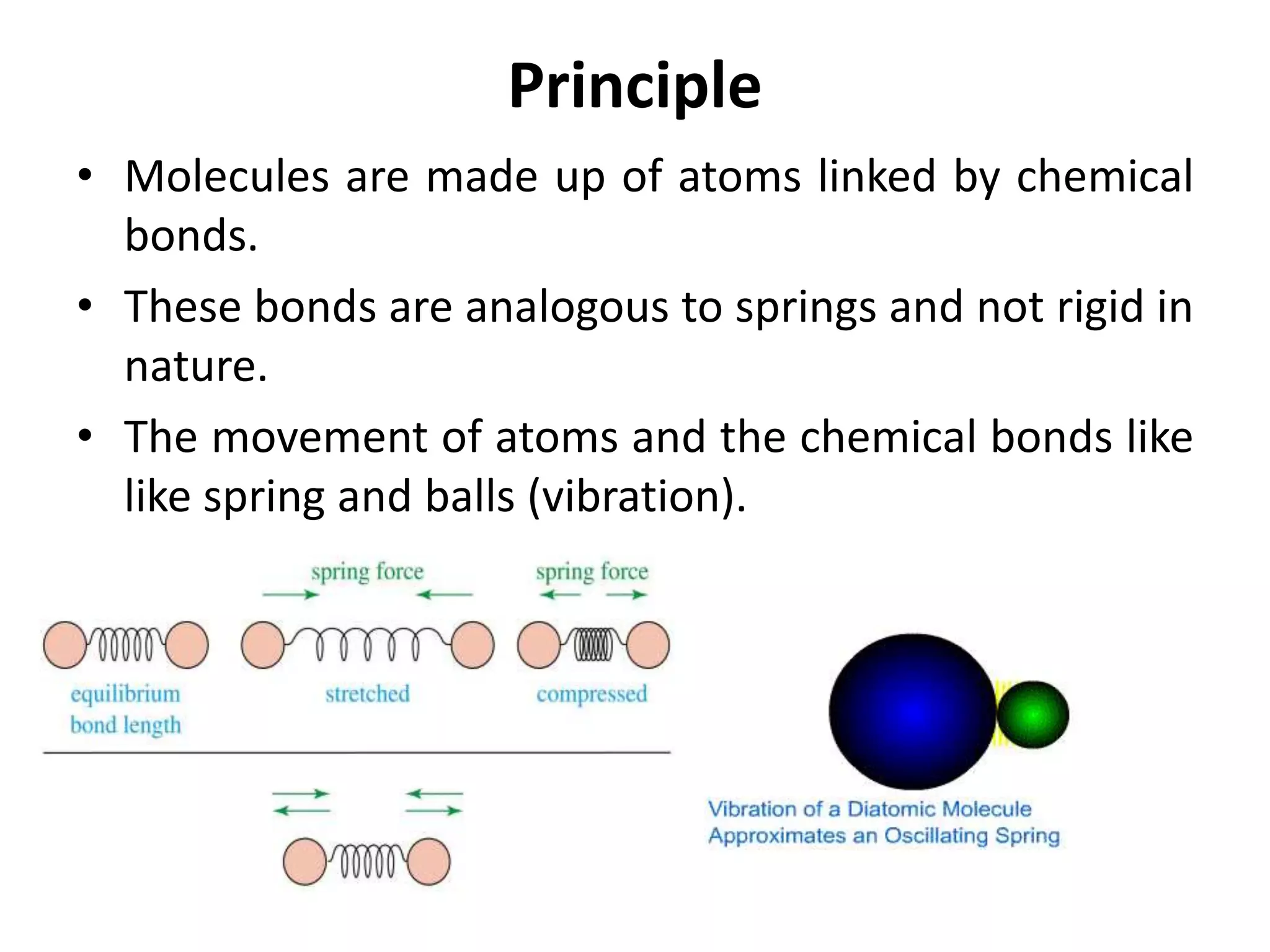
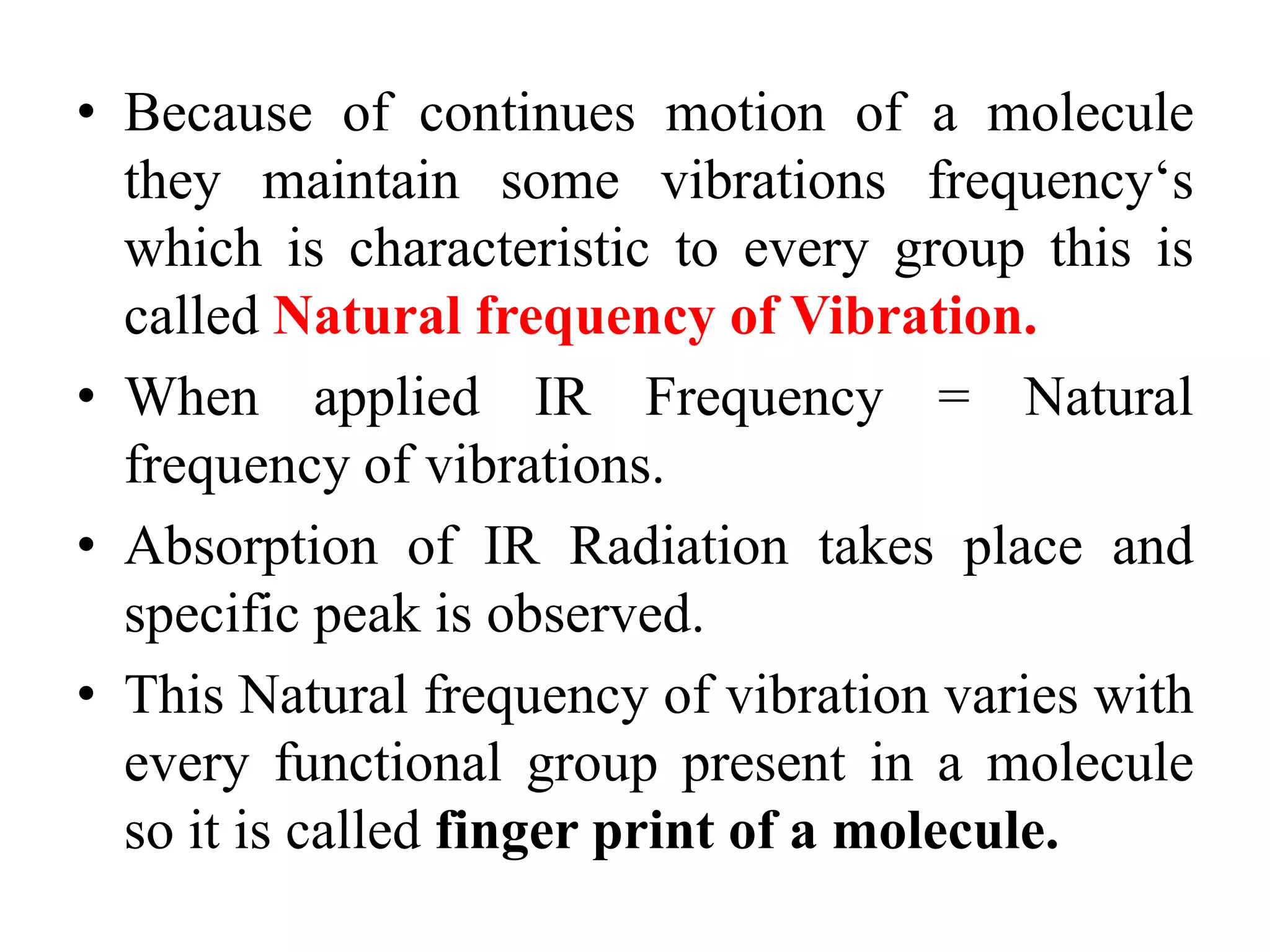
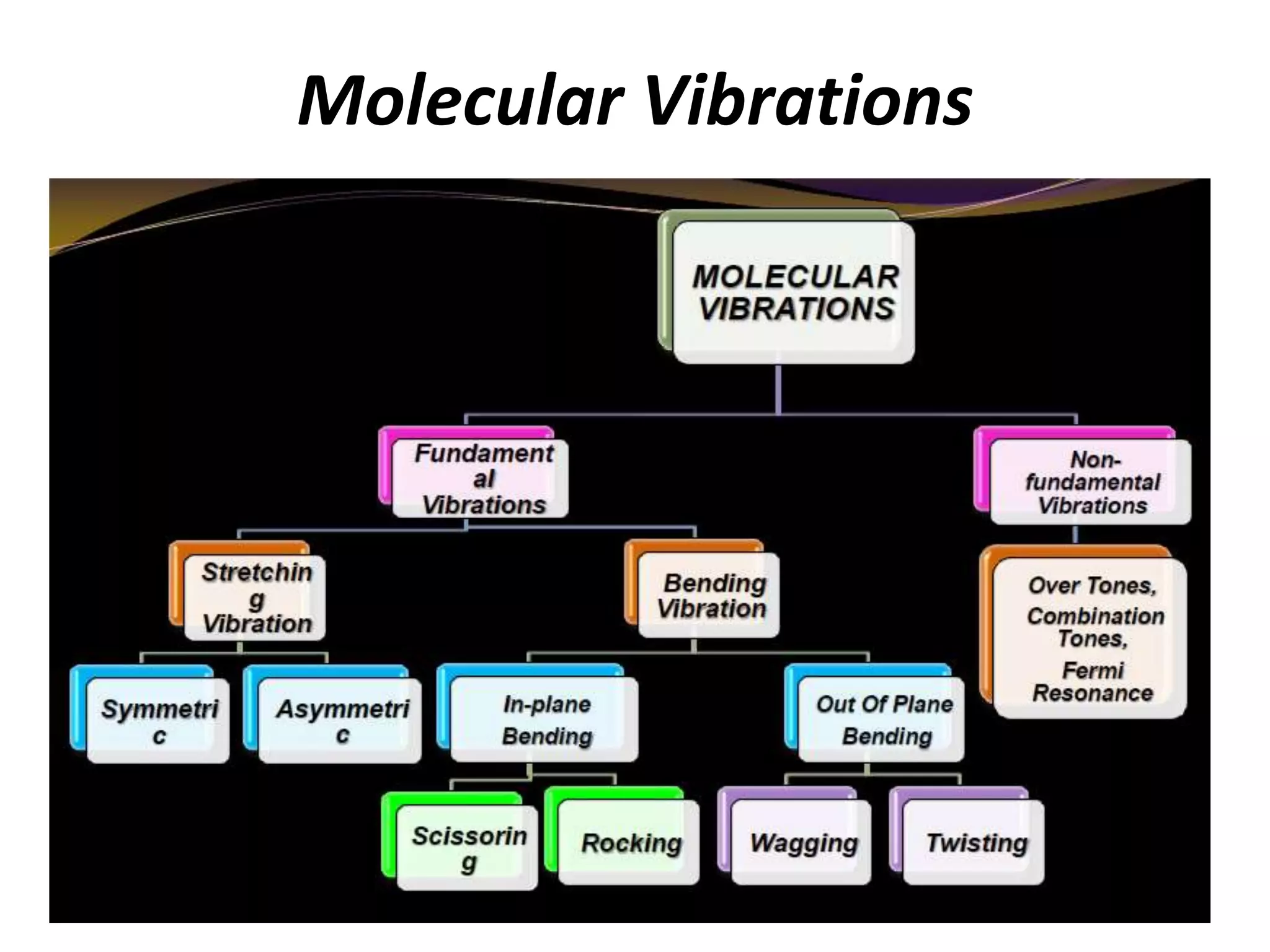
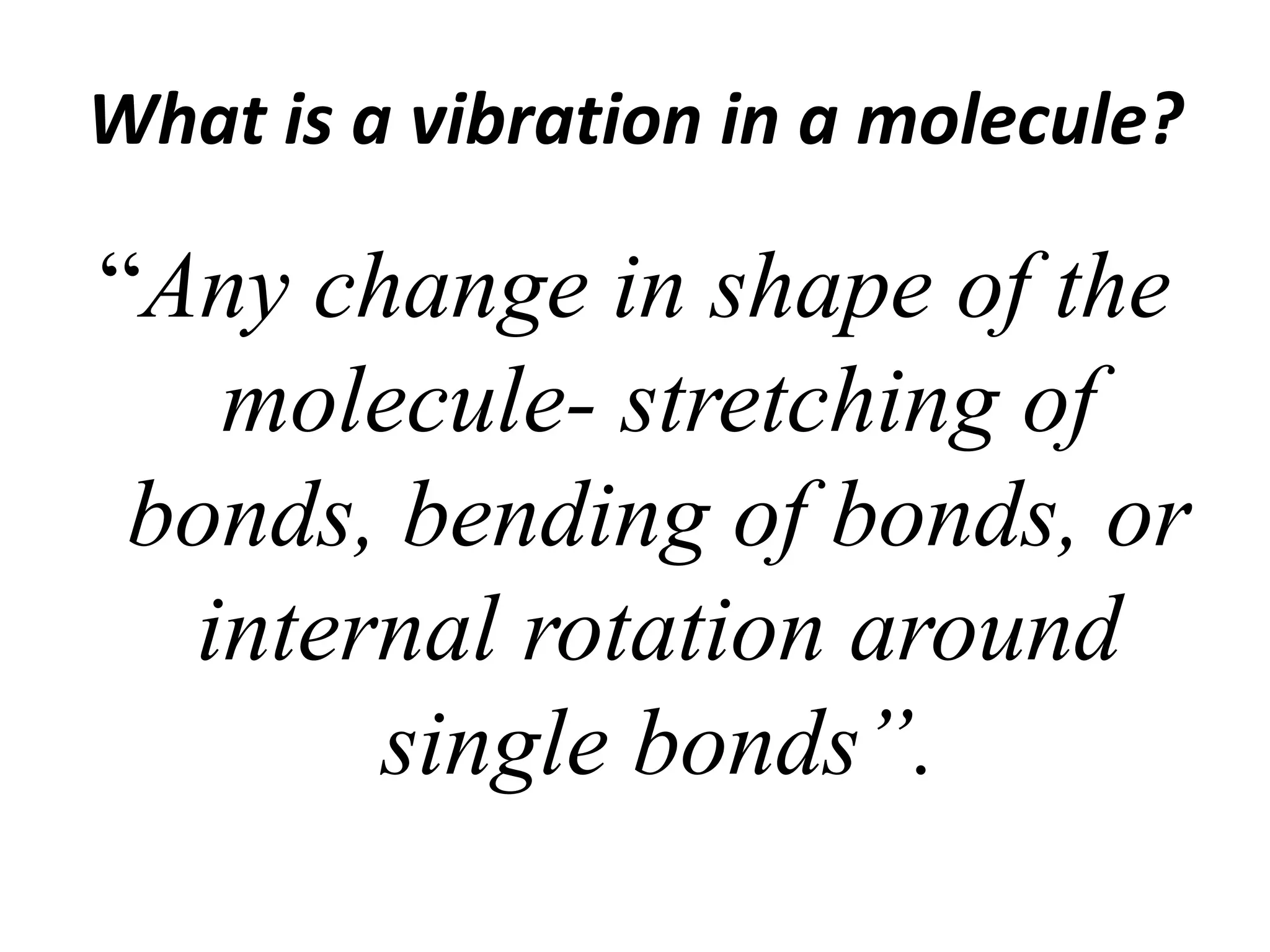
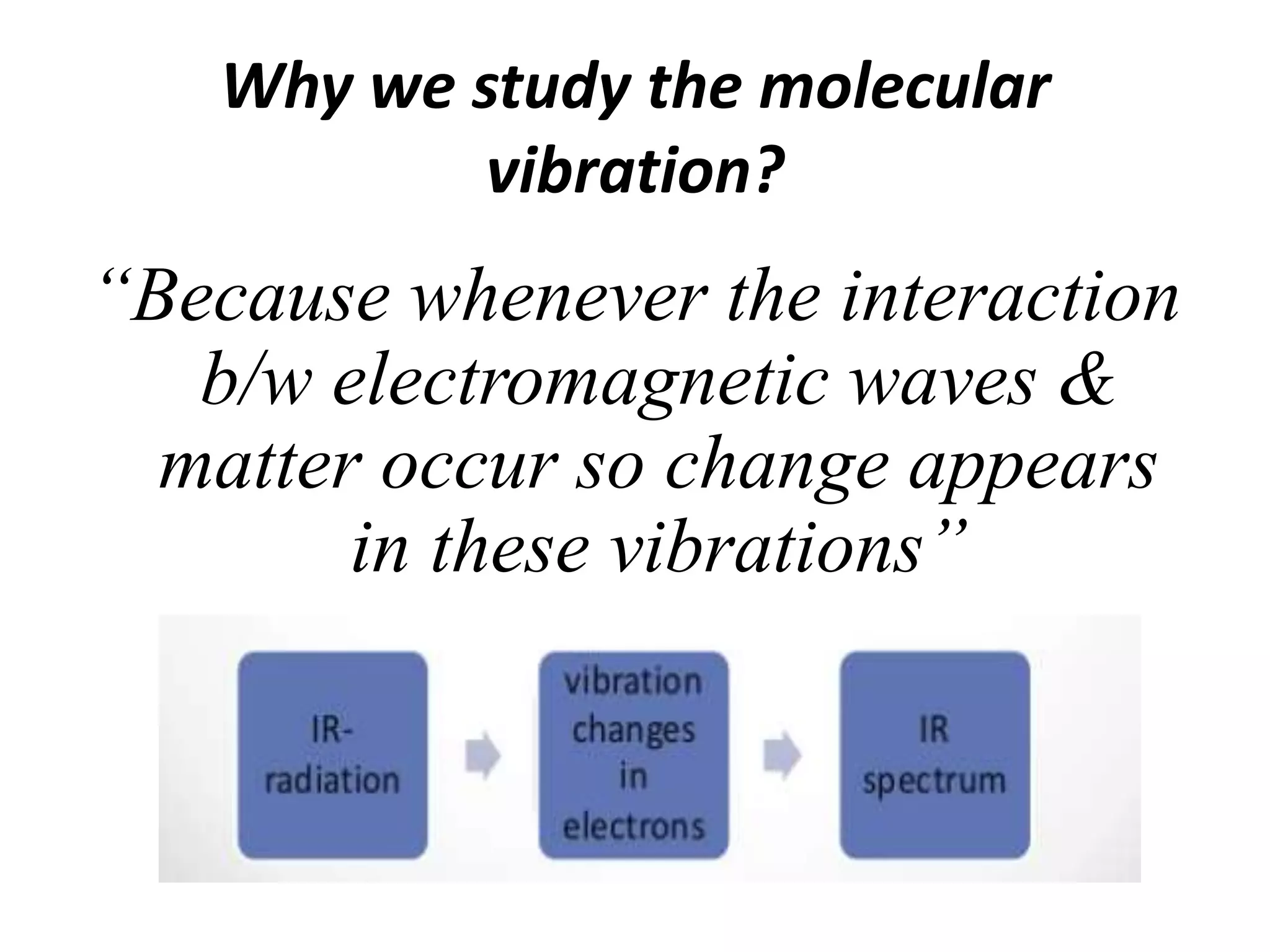
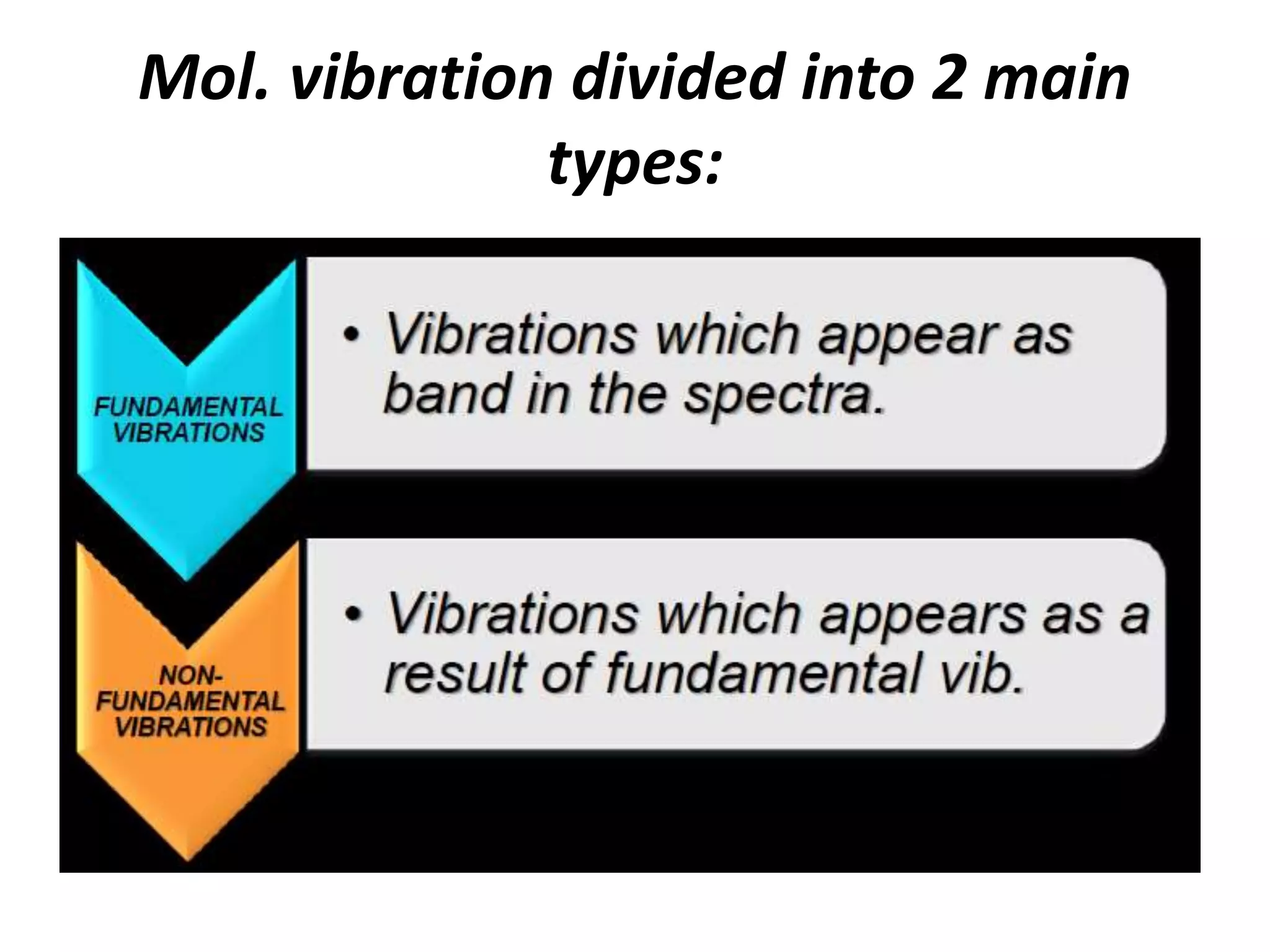
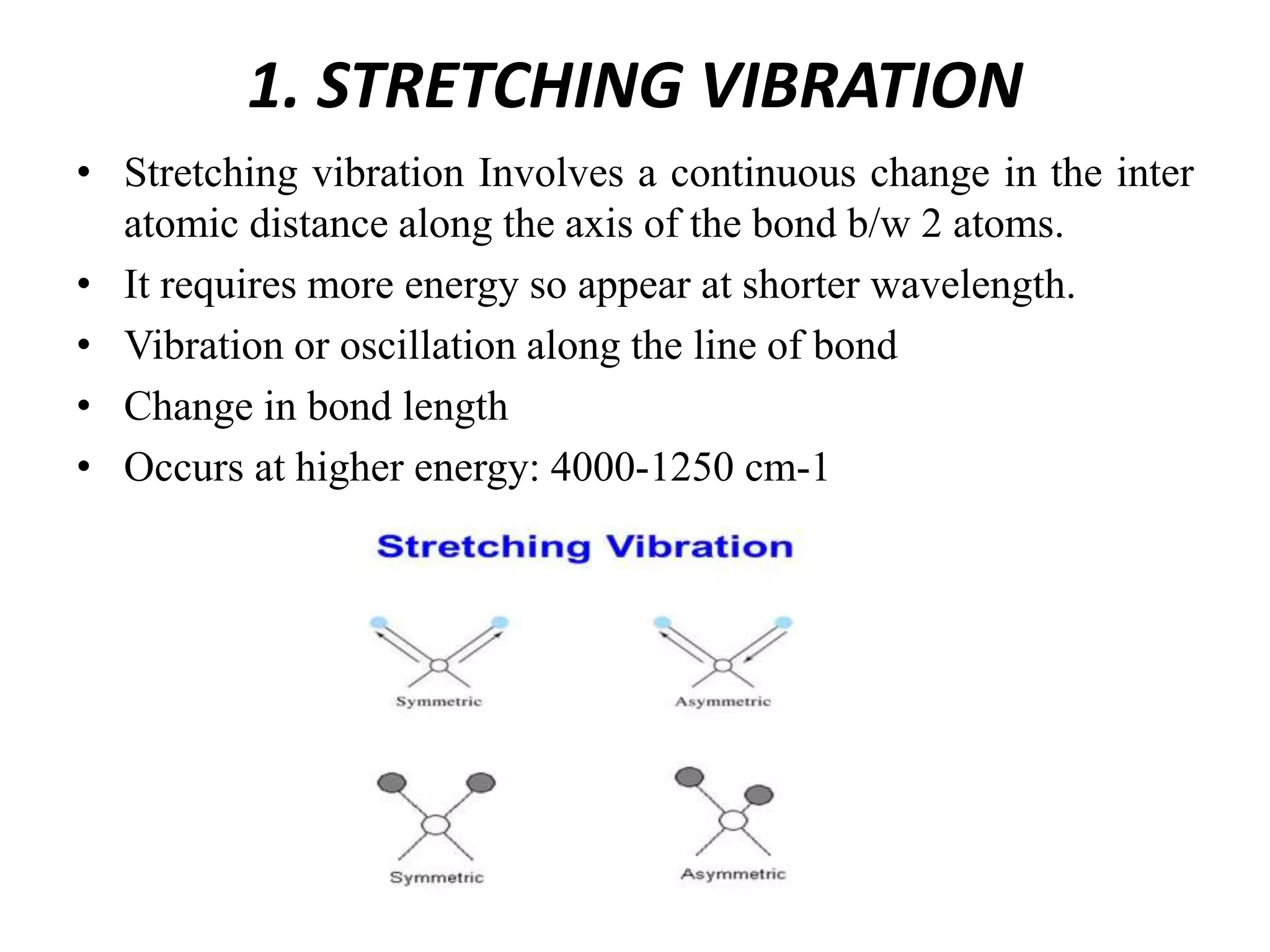
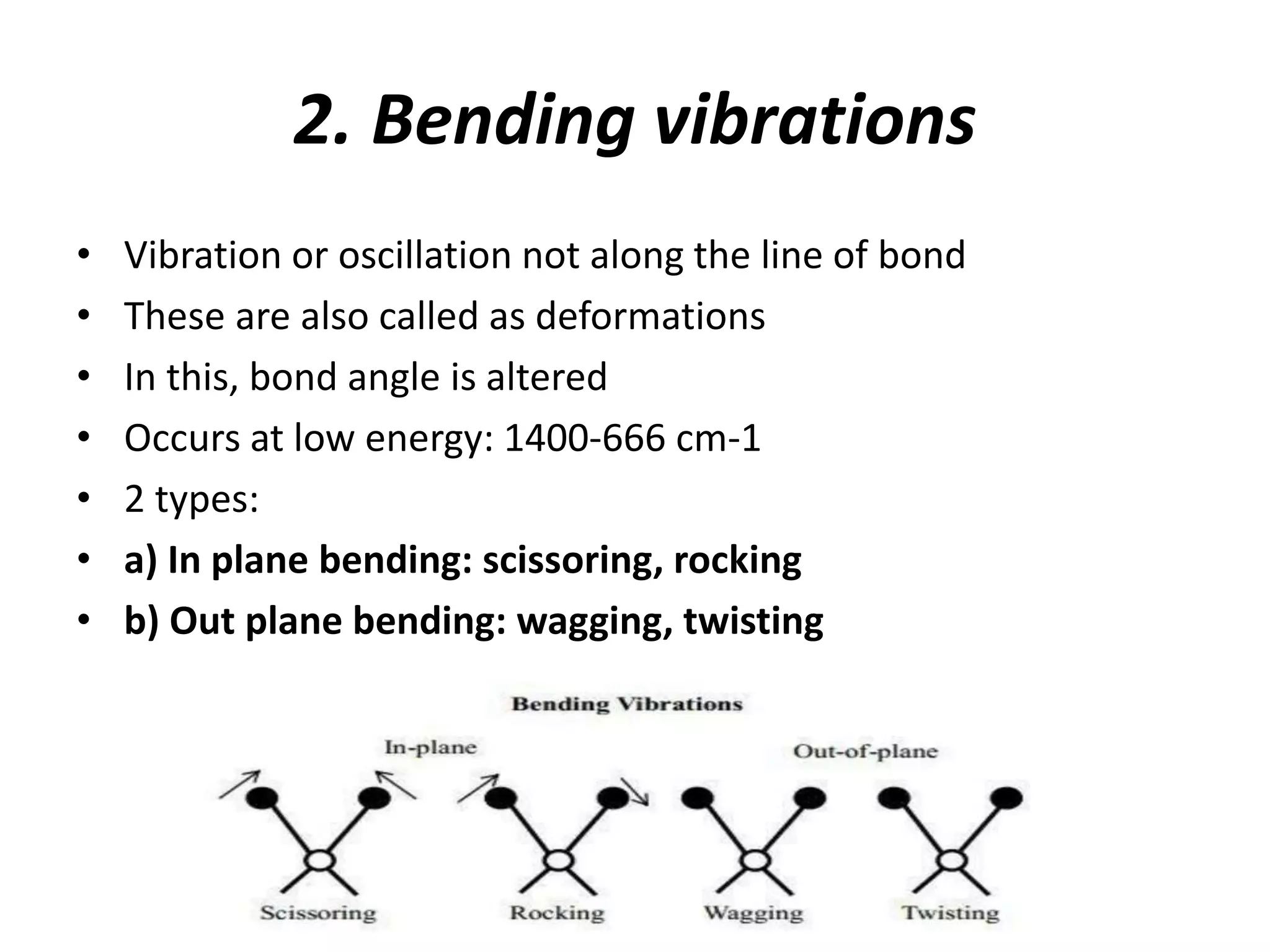
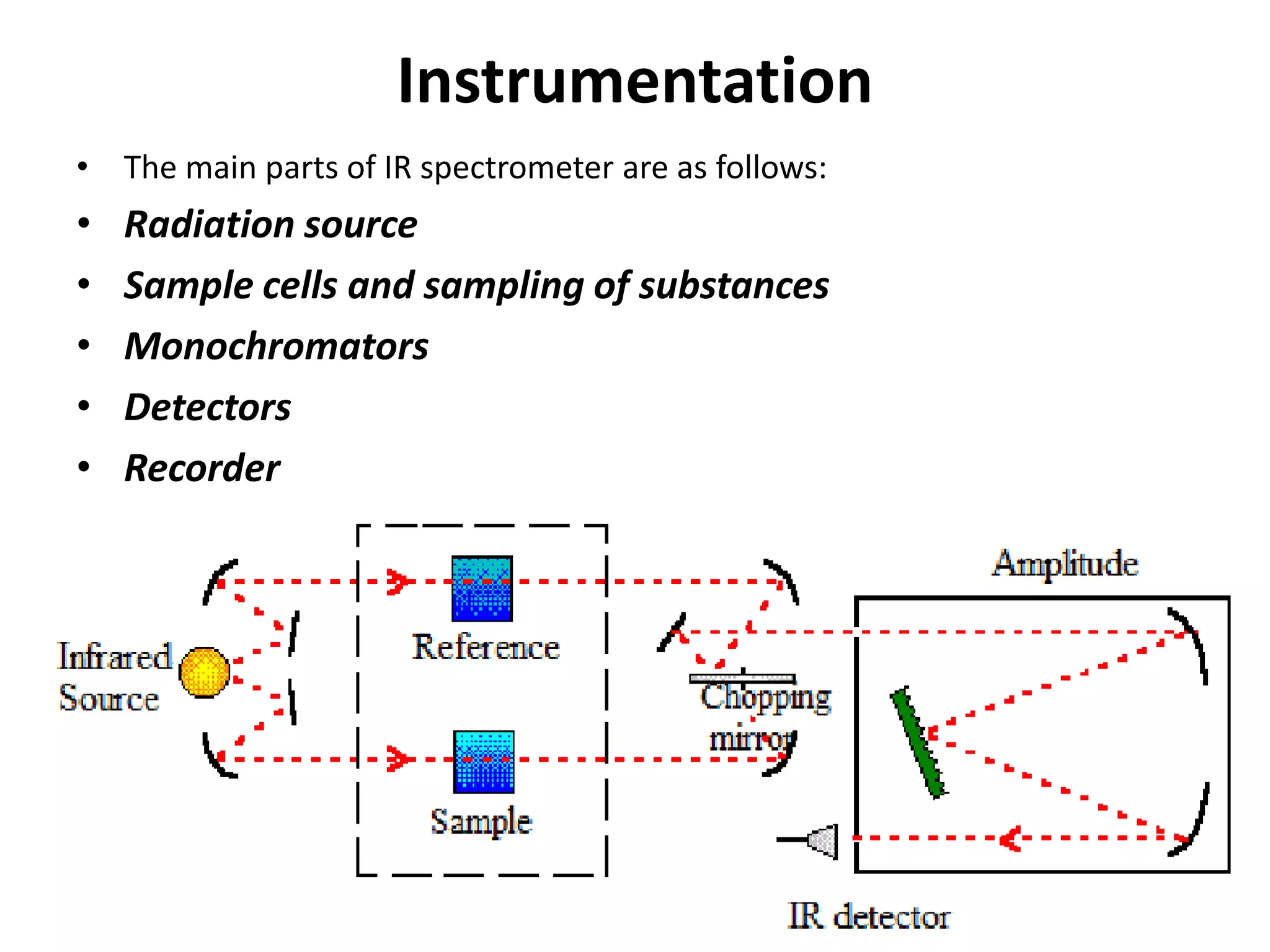
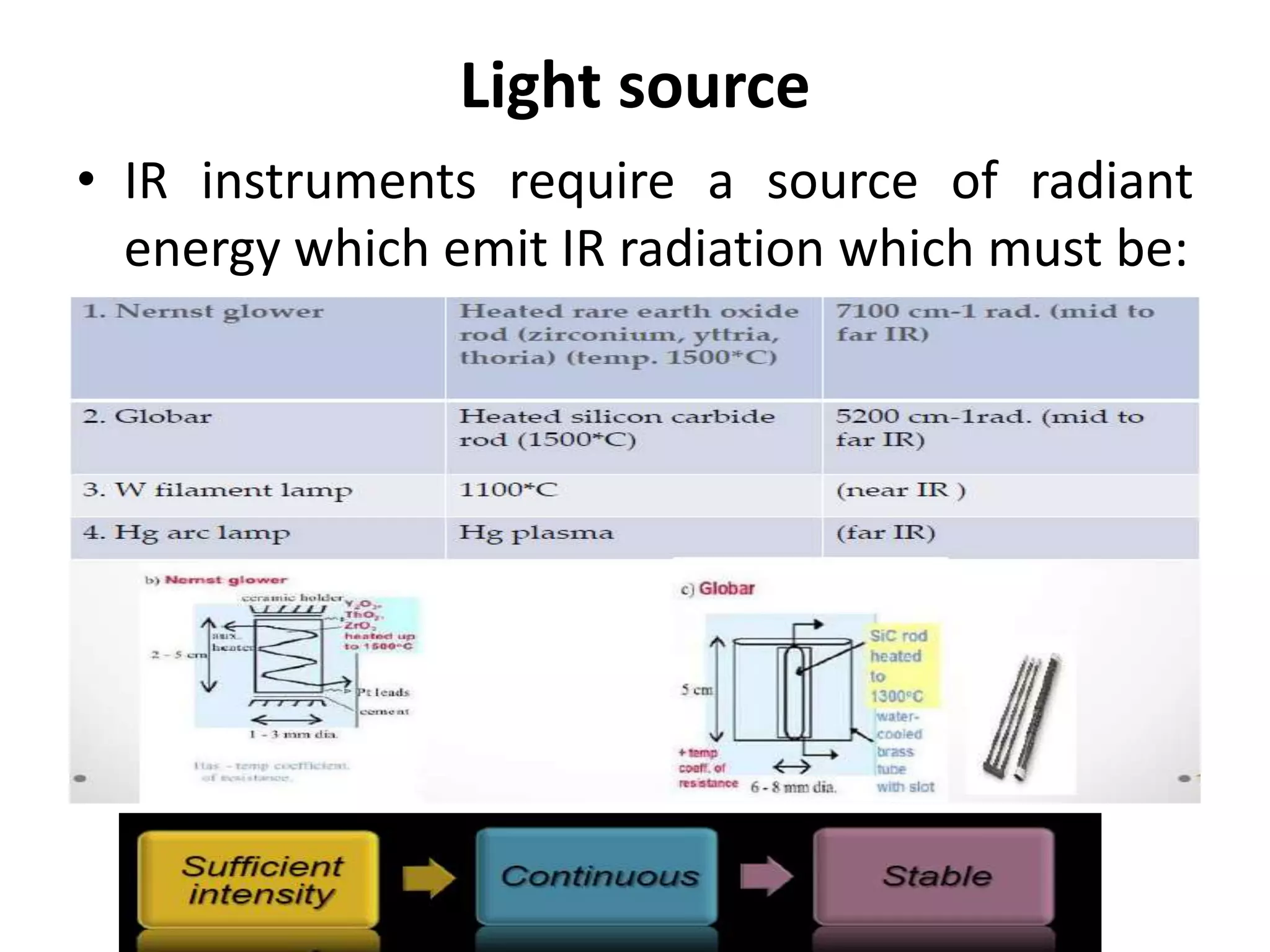
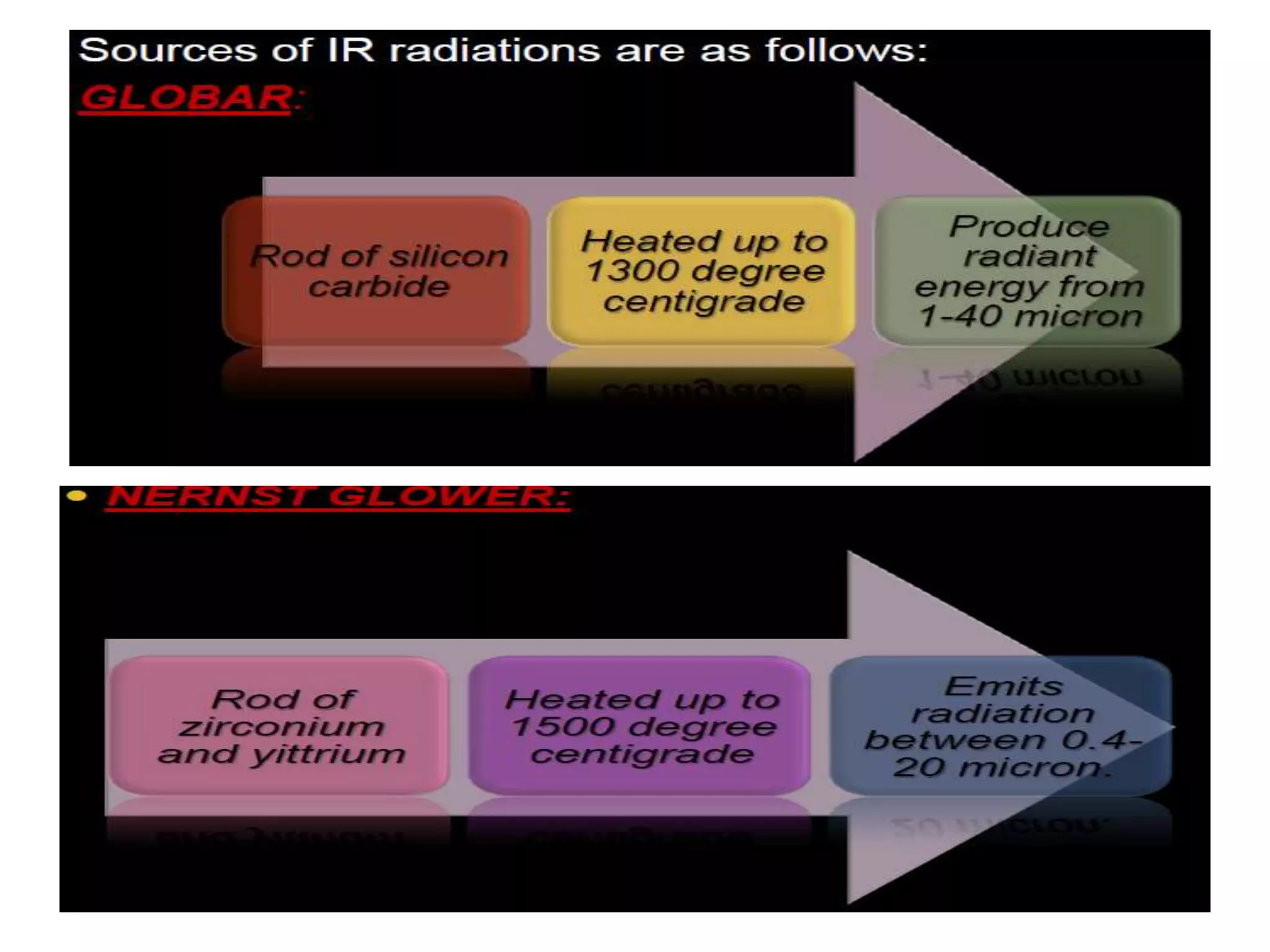
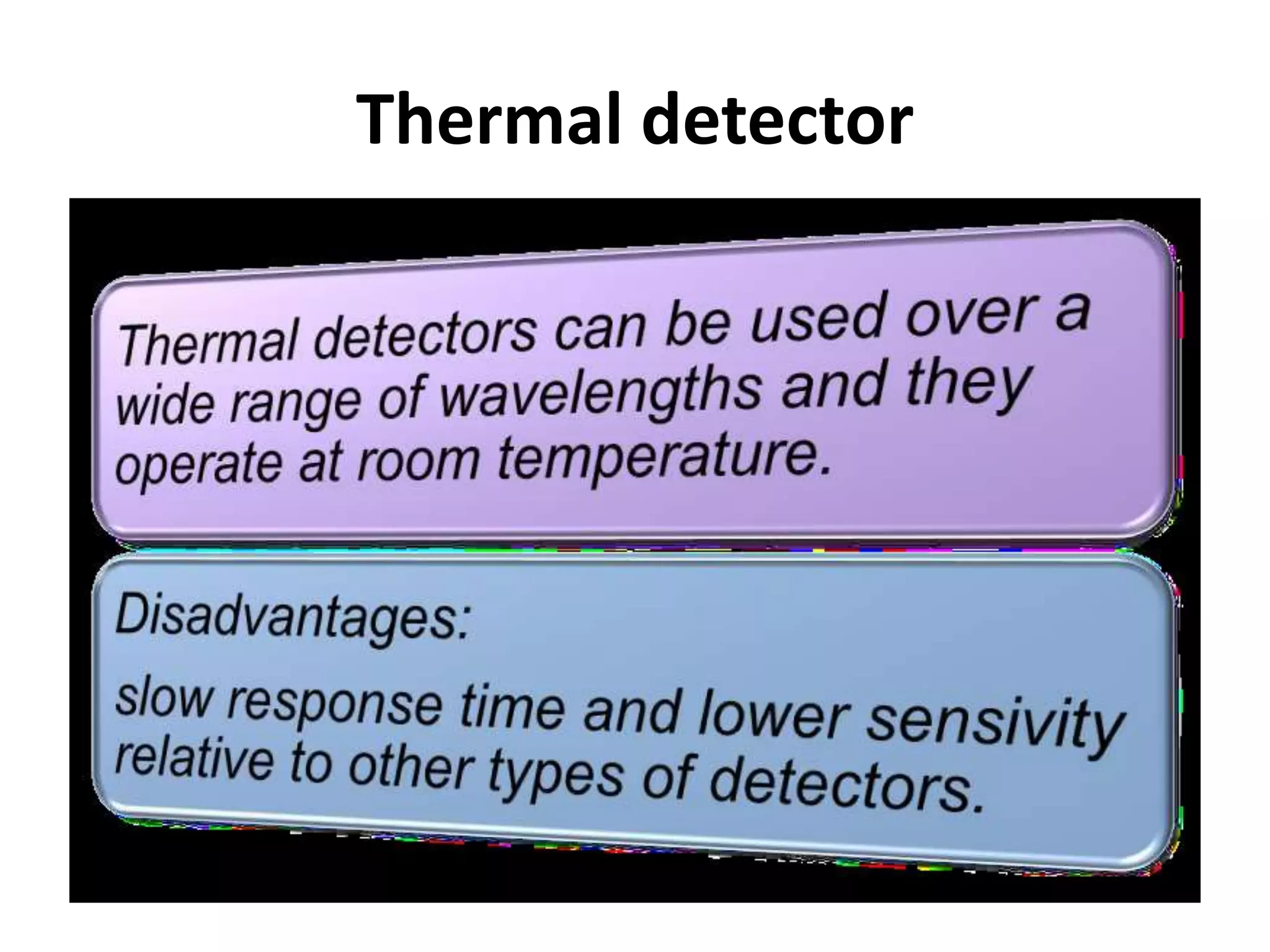
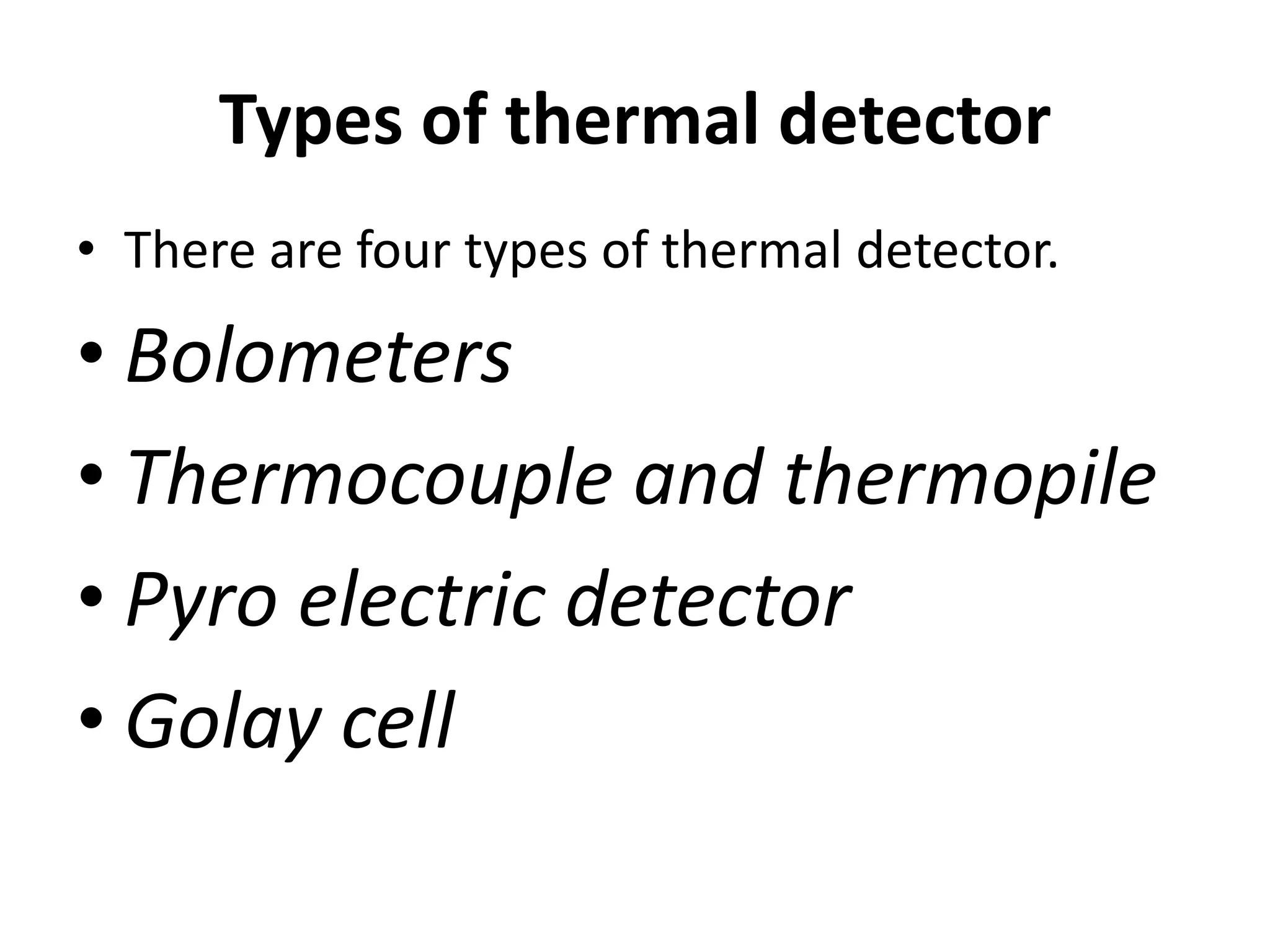
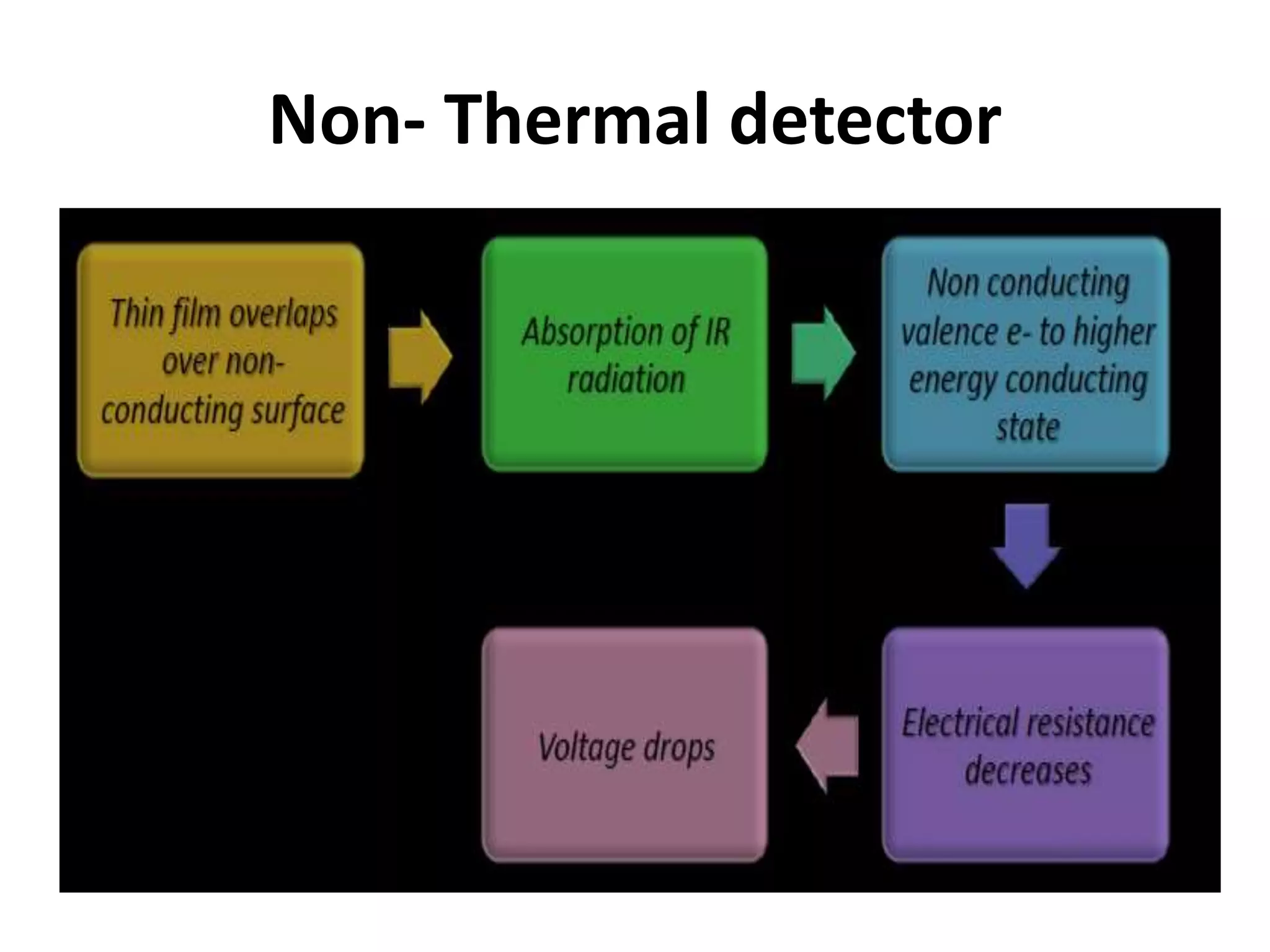
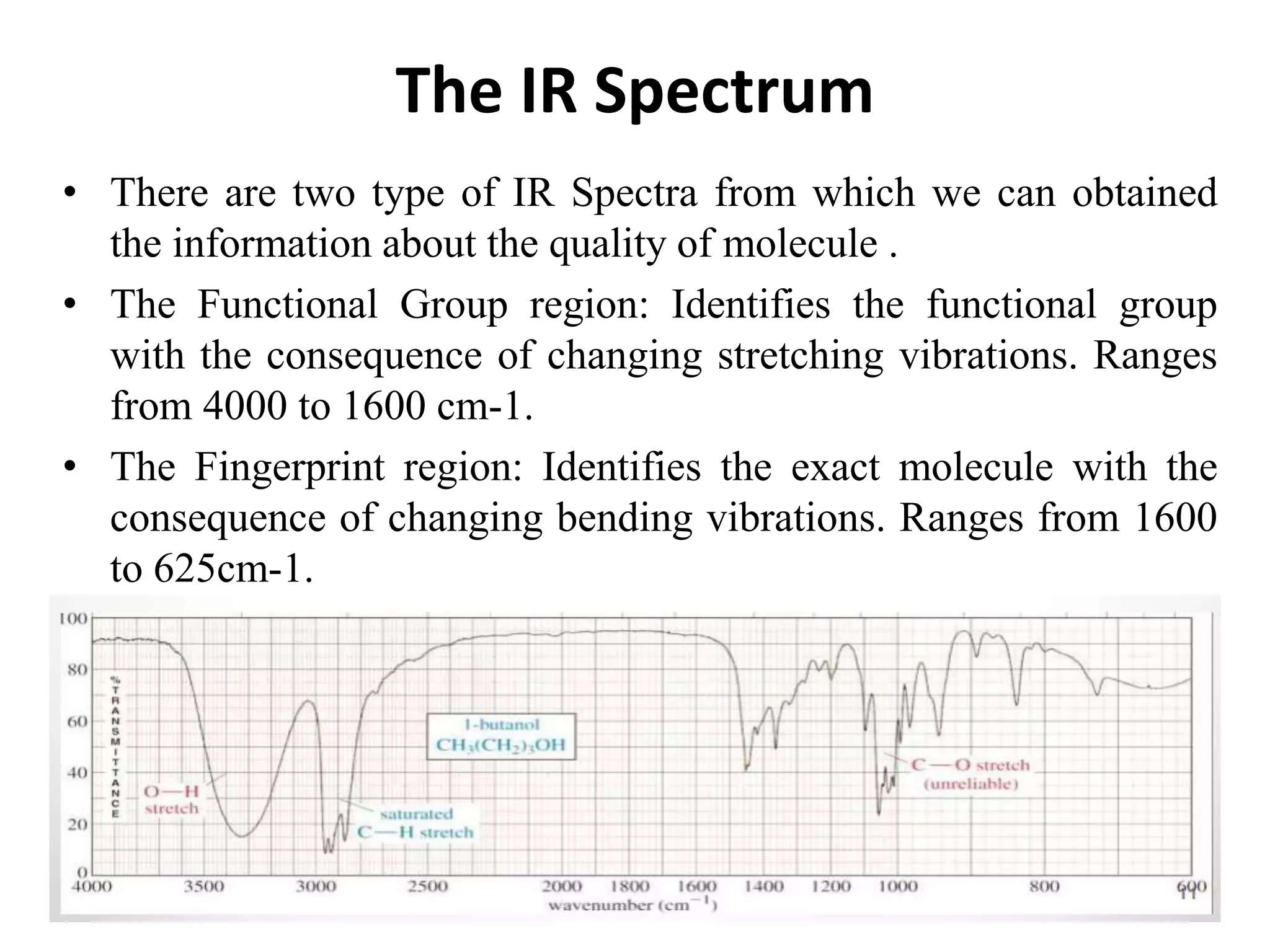
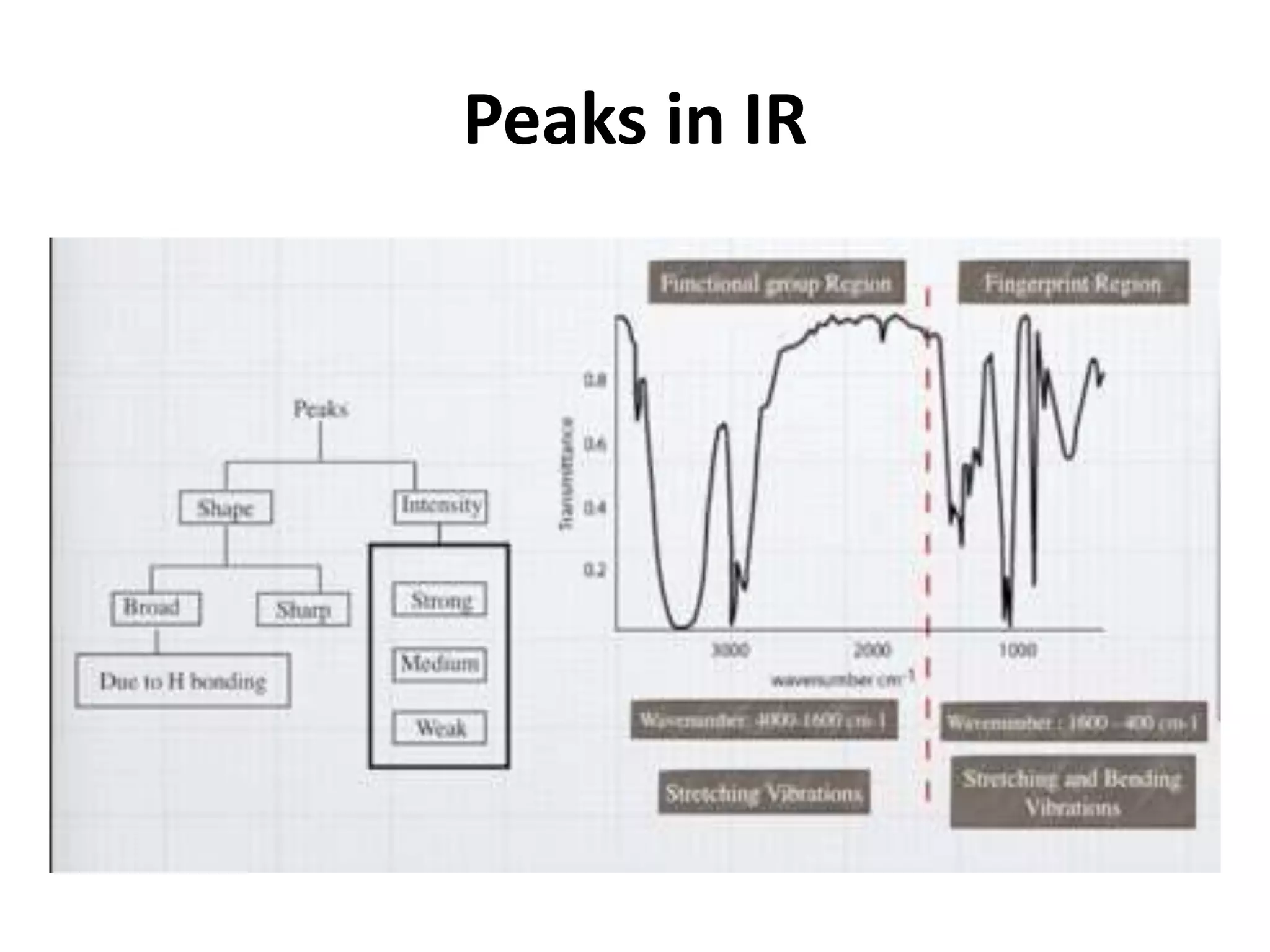
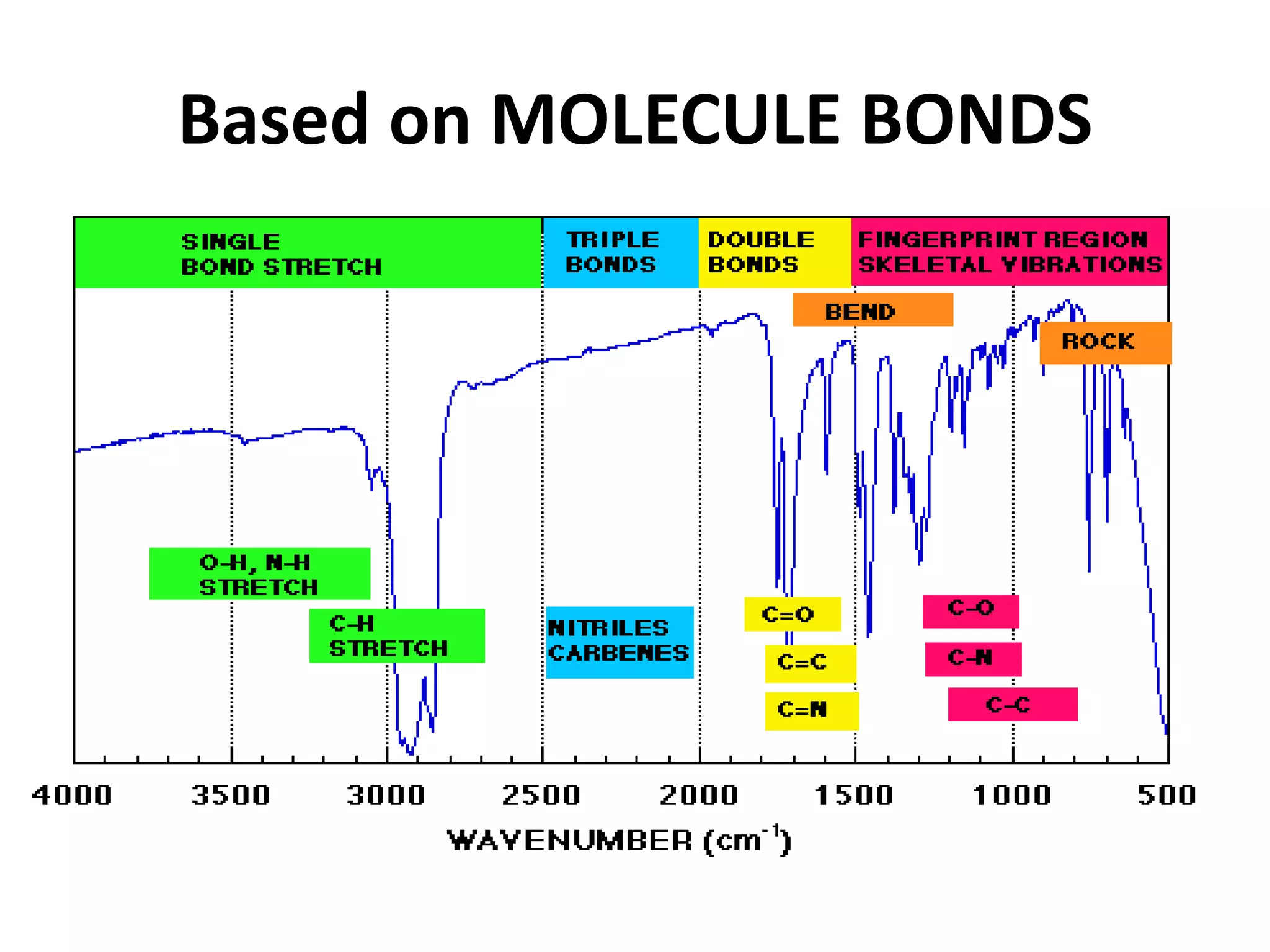
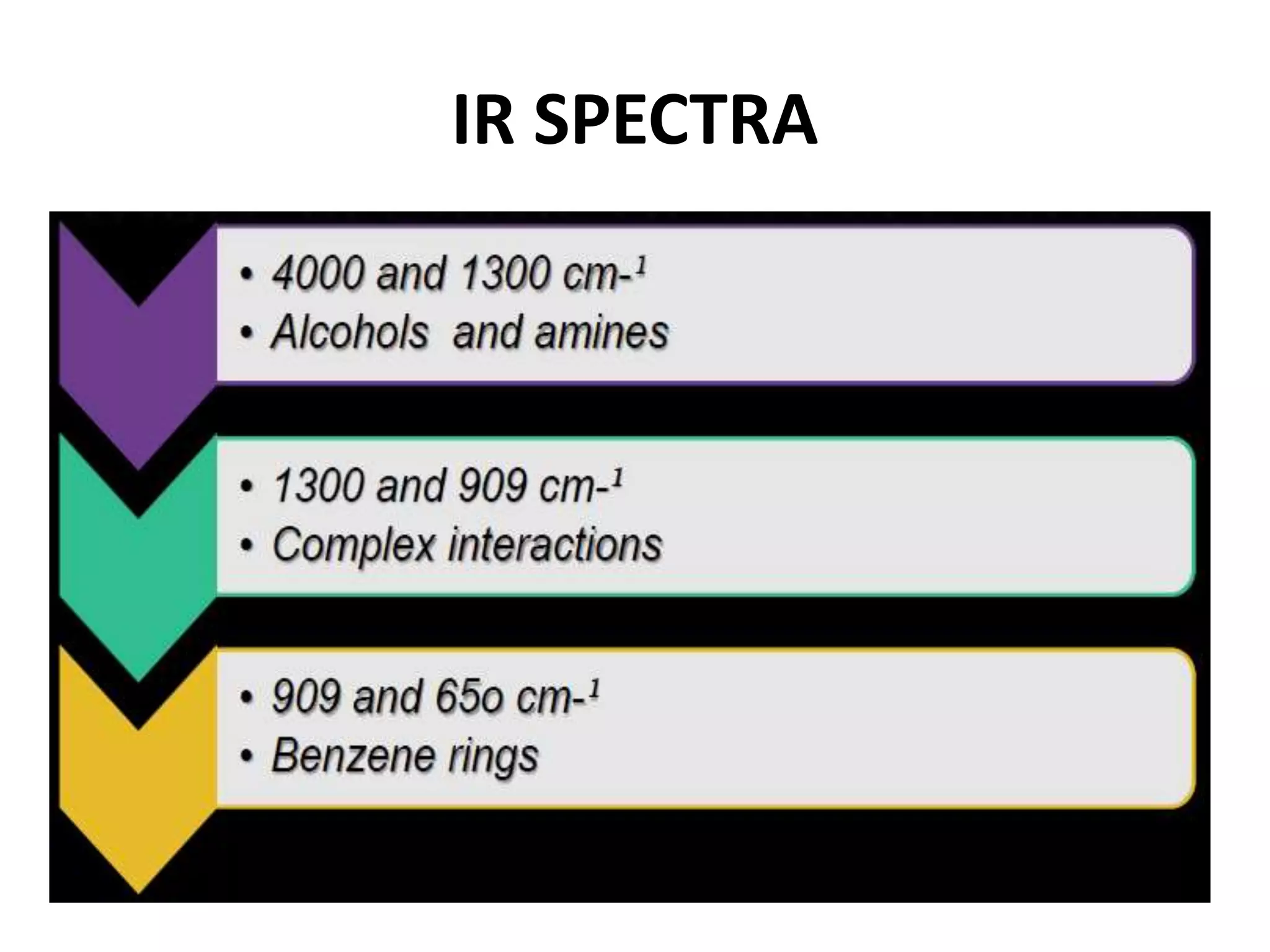
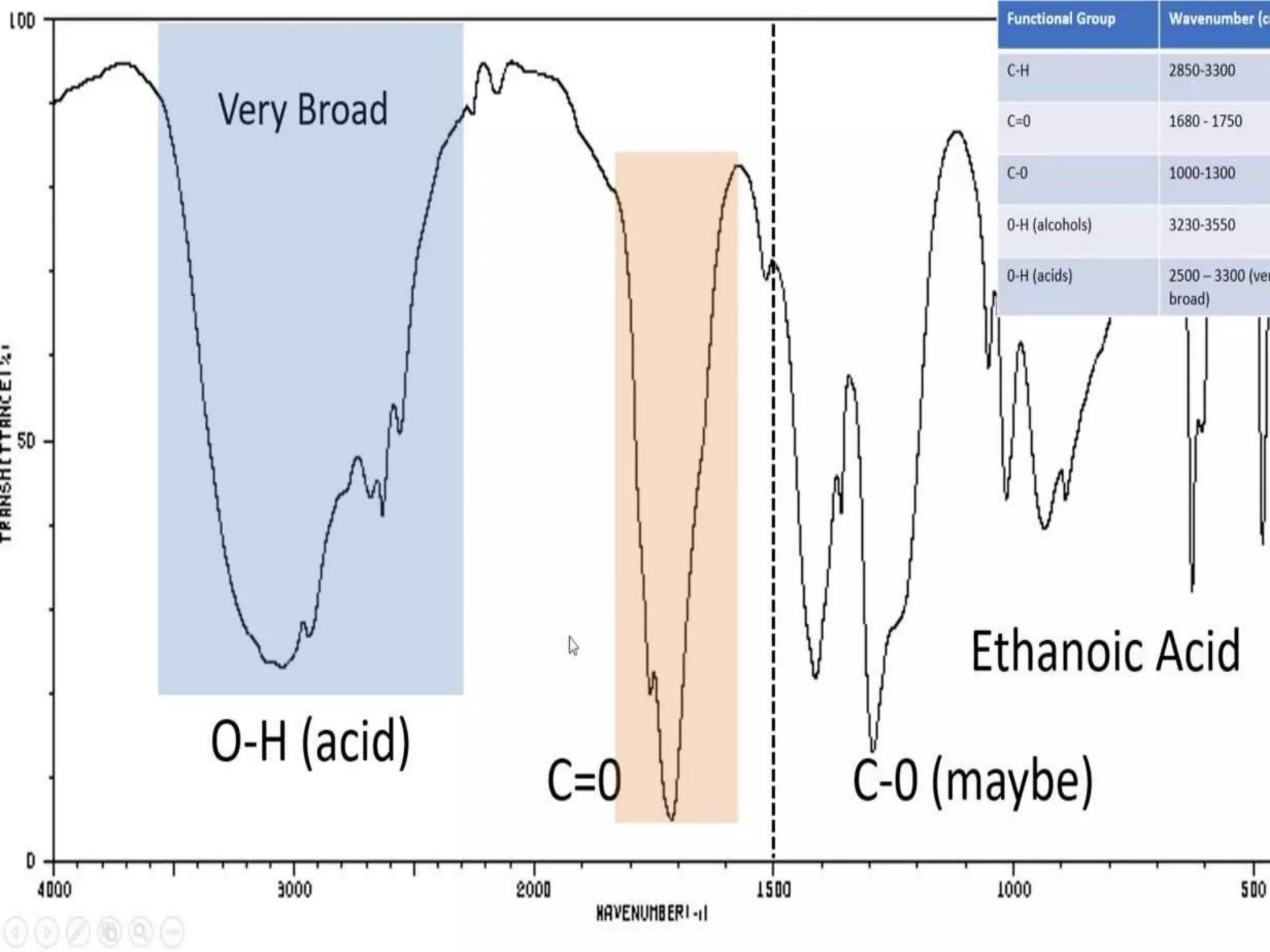
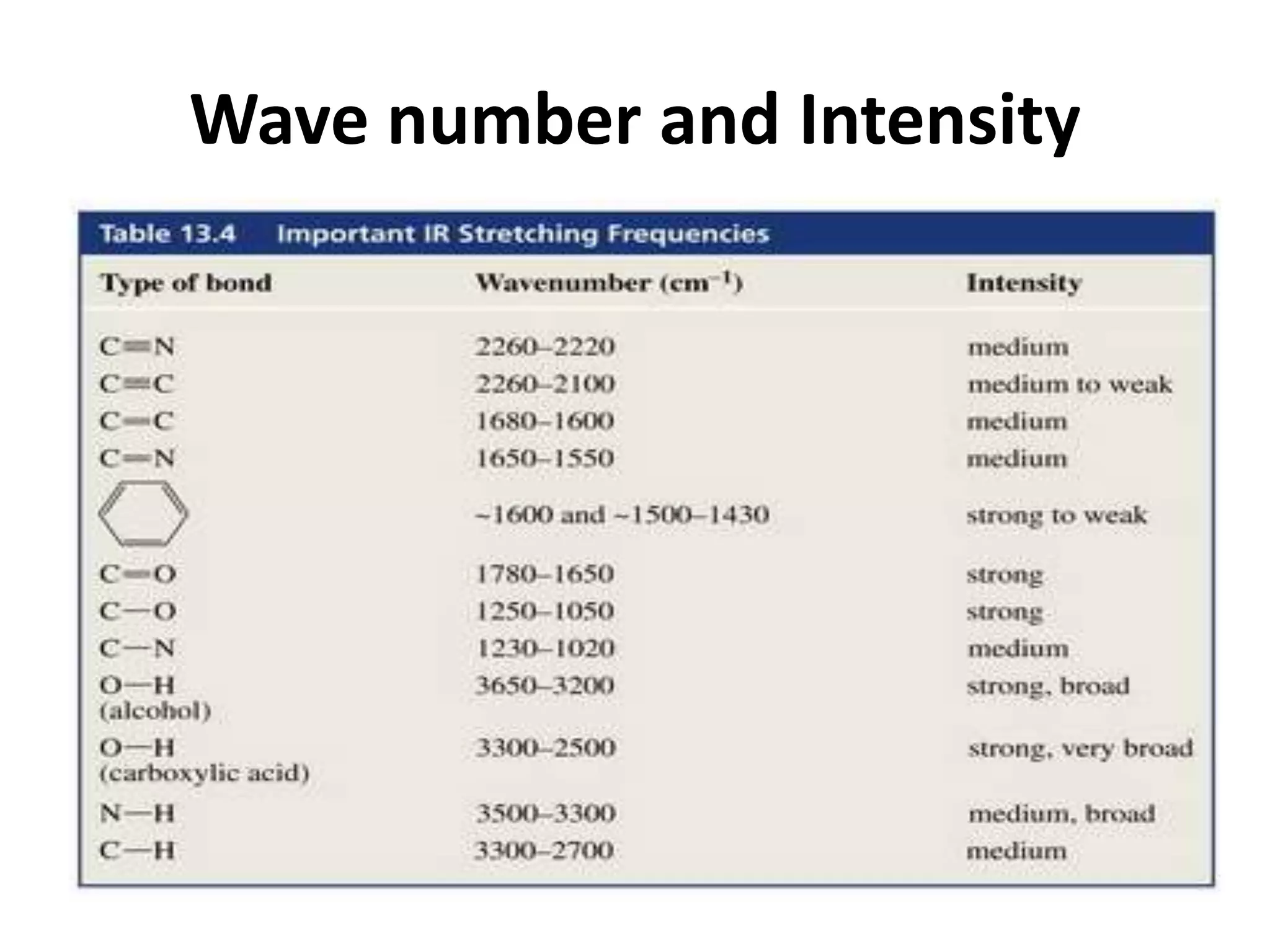
Infrared spectroscopy analyzes the absorption of infrared light by molecules to determine their structure. It is mainly concerned with studying vibrational transitions that occur when the wavelength of infrared radiation matches the natural frequency of vibration of bonds in a molecule. Infrared spectroscopy can be used to identify functional groups and determine the structure of organic compounds. The infrared spectrum is divided into functional group and fingerprint regions that provide information about bond vibrations and molecular structure.