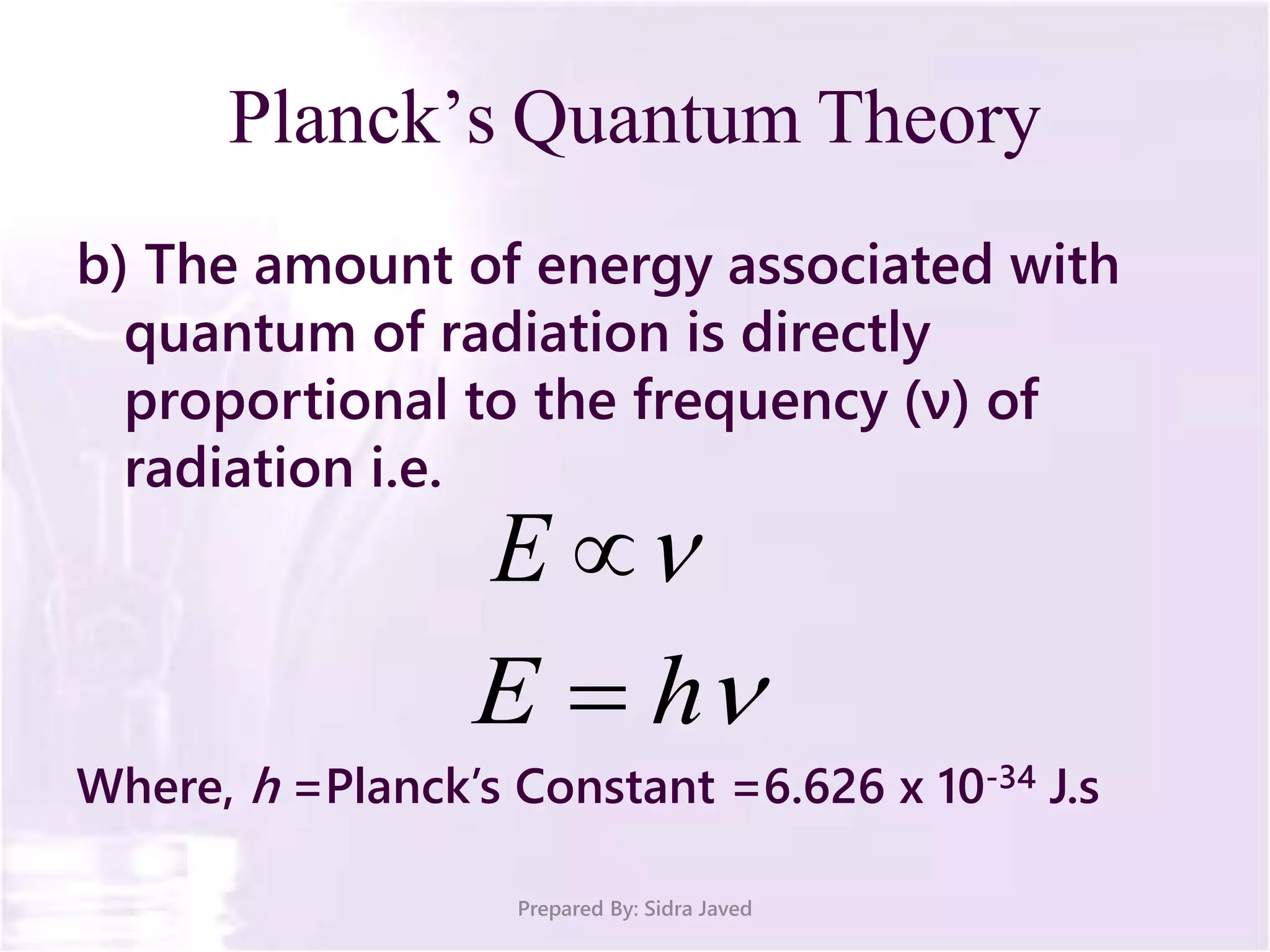
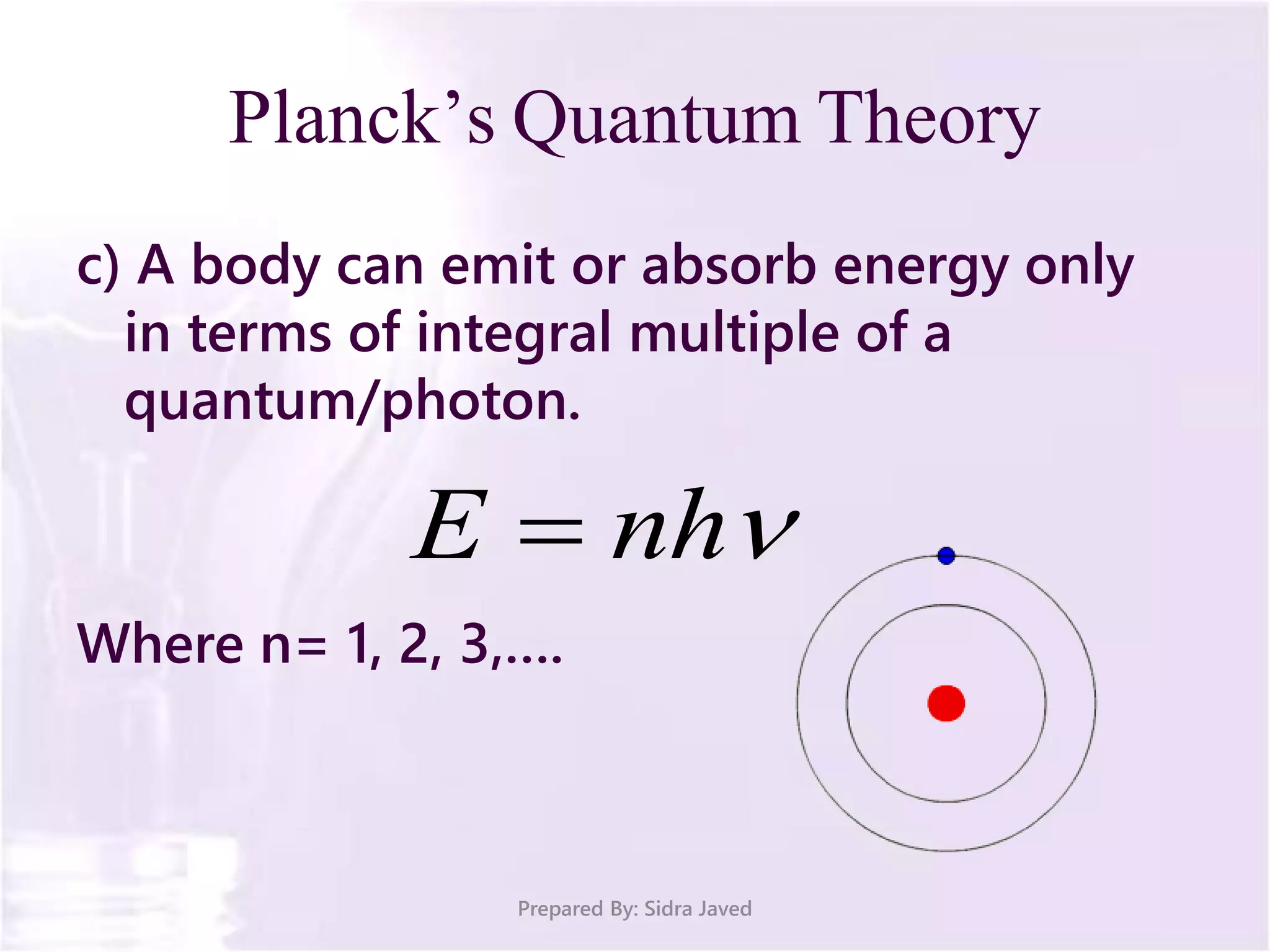
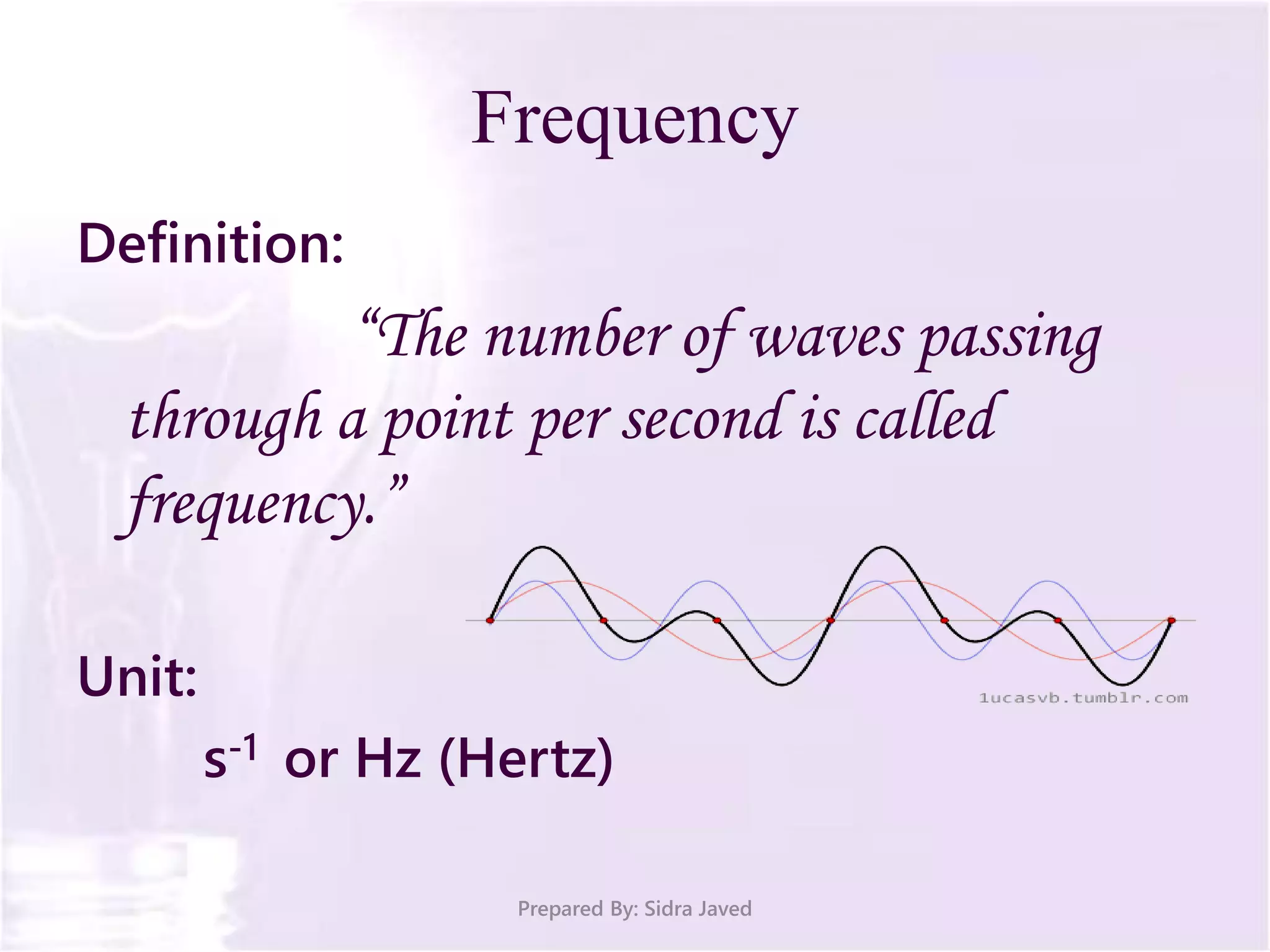
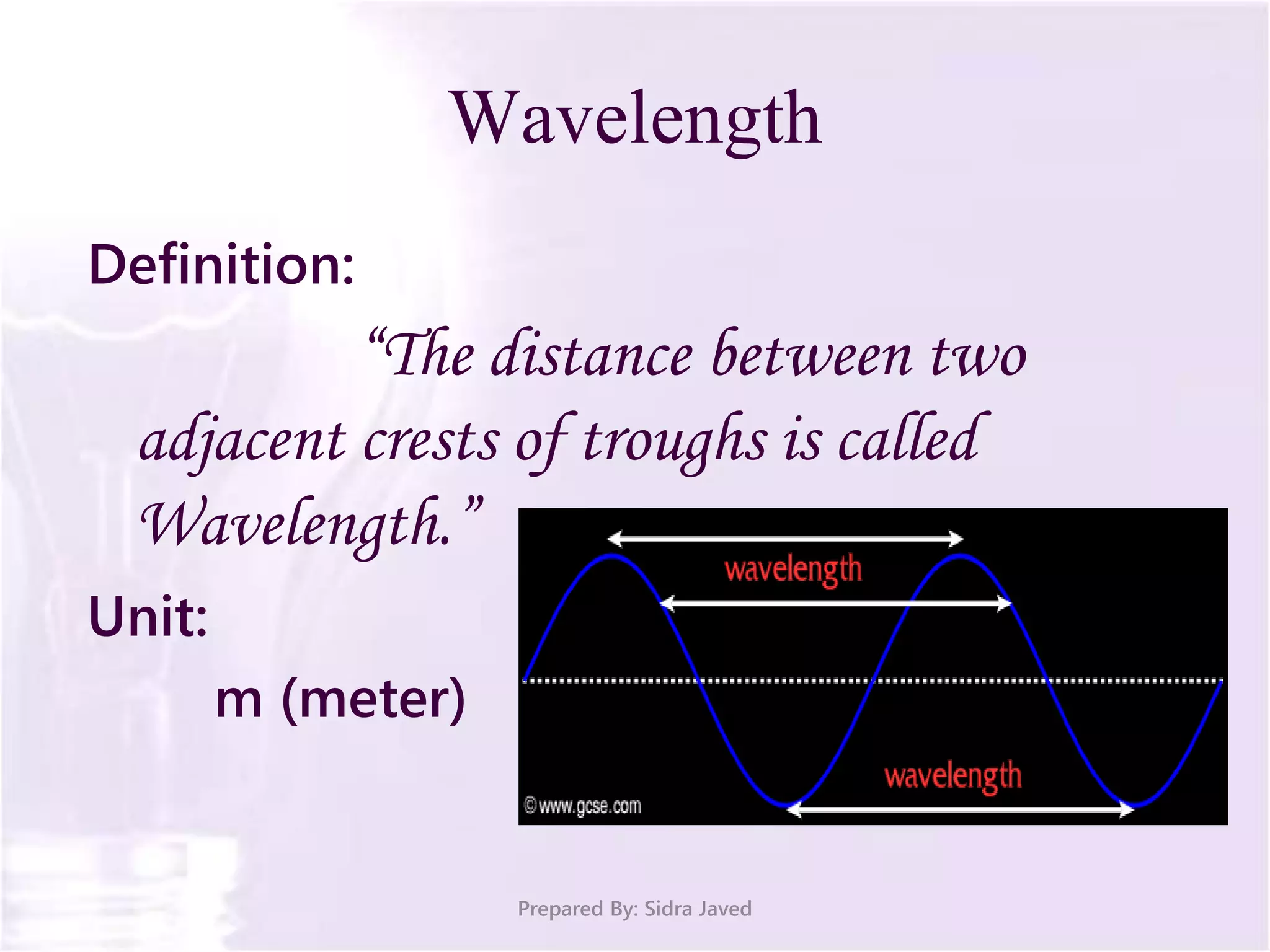
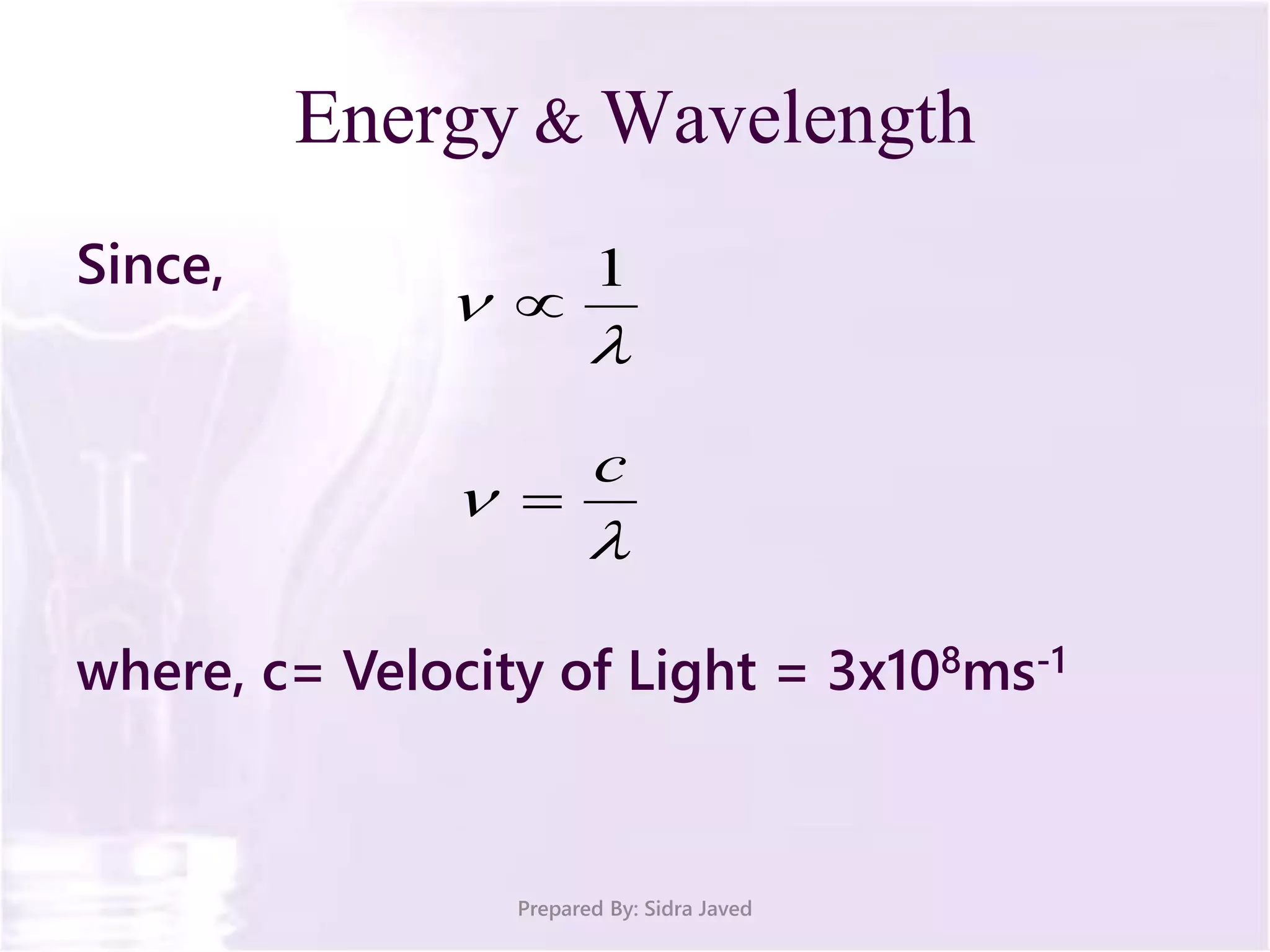
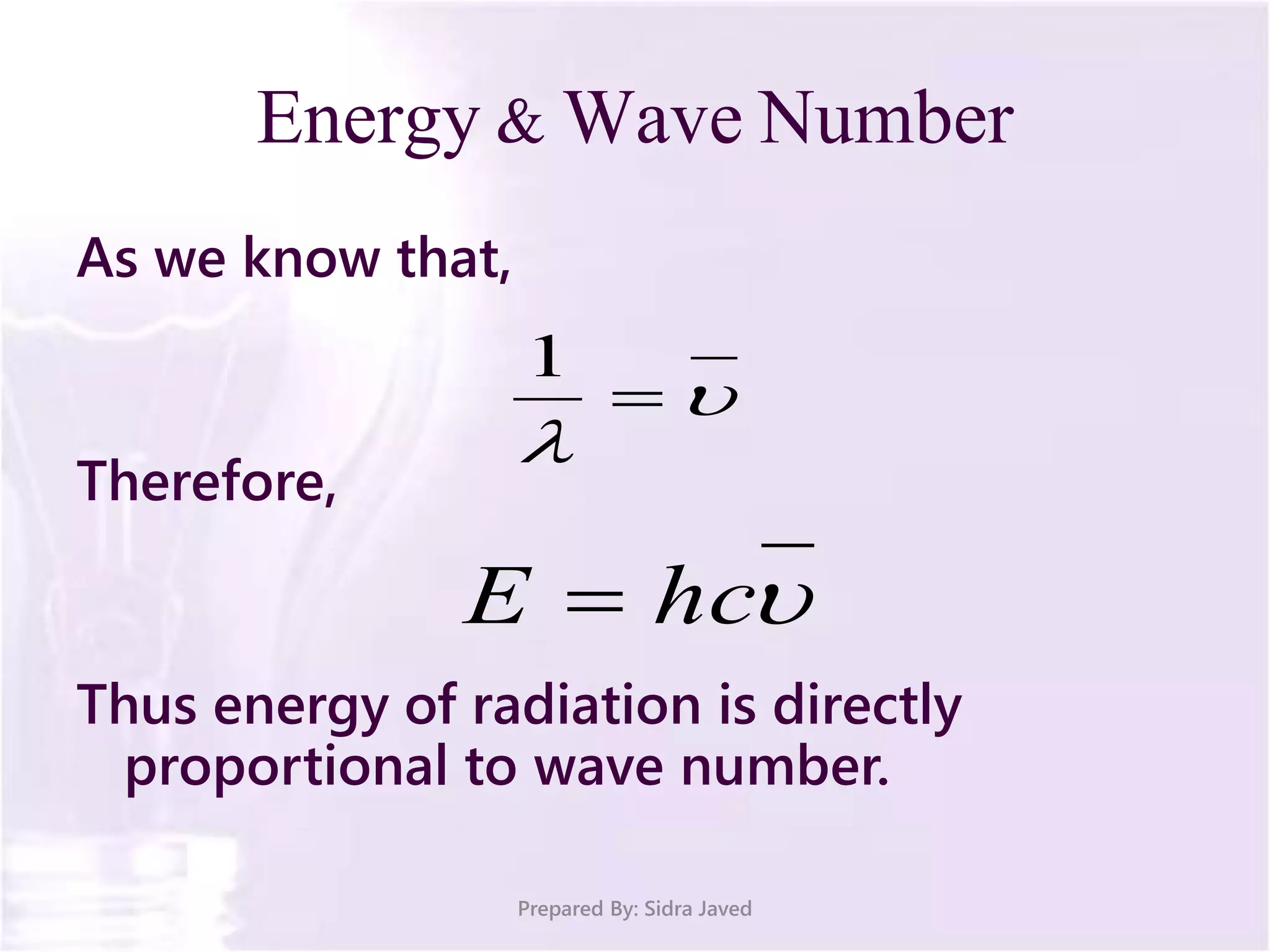
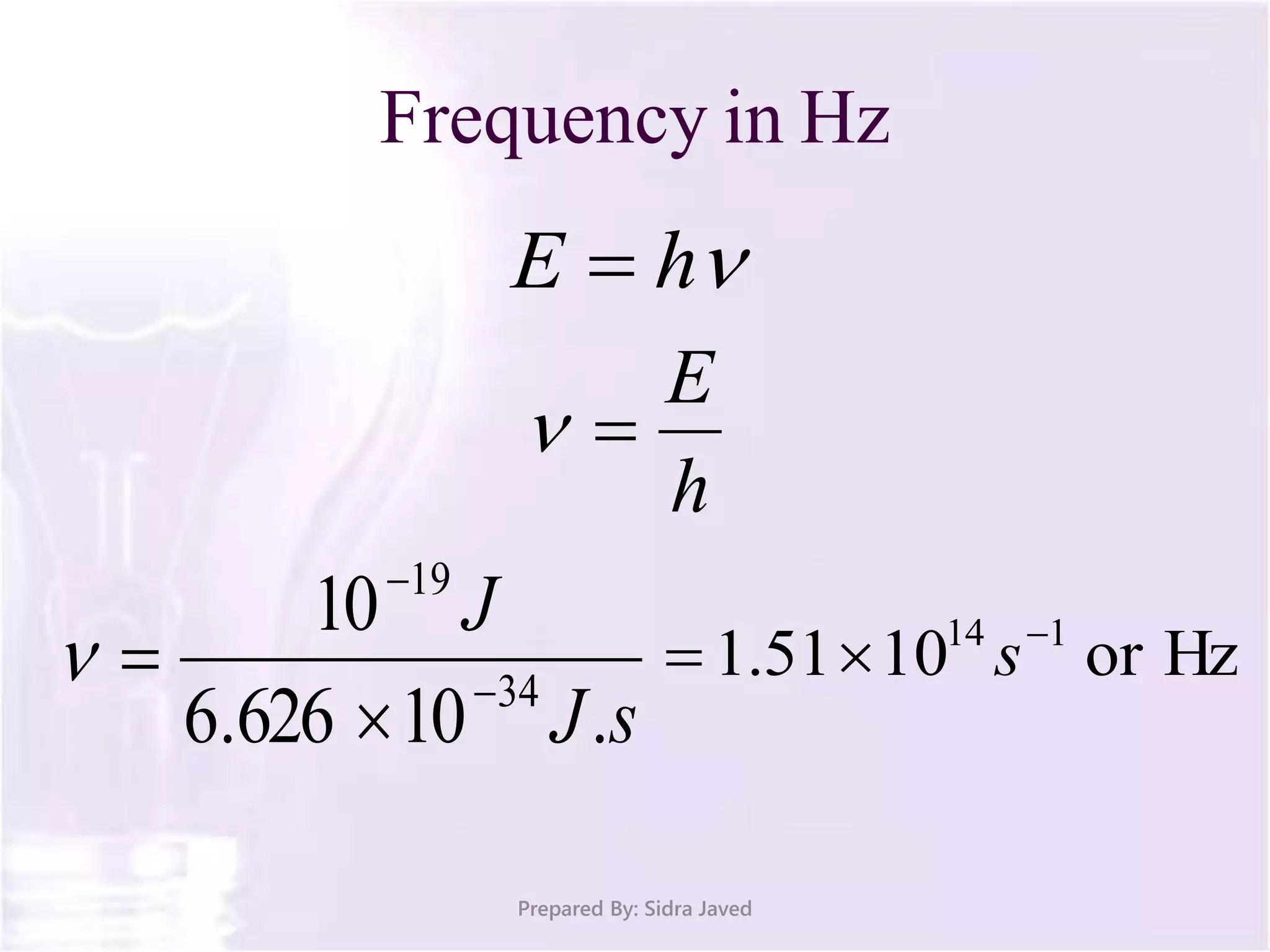
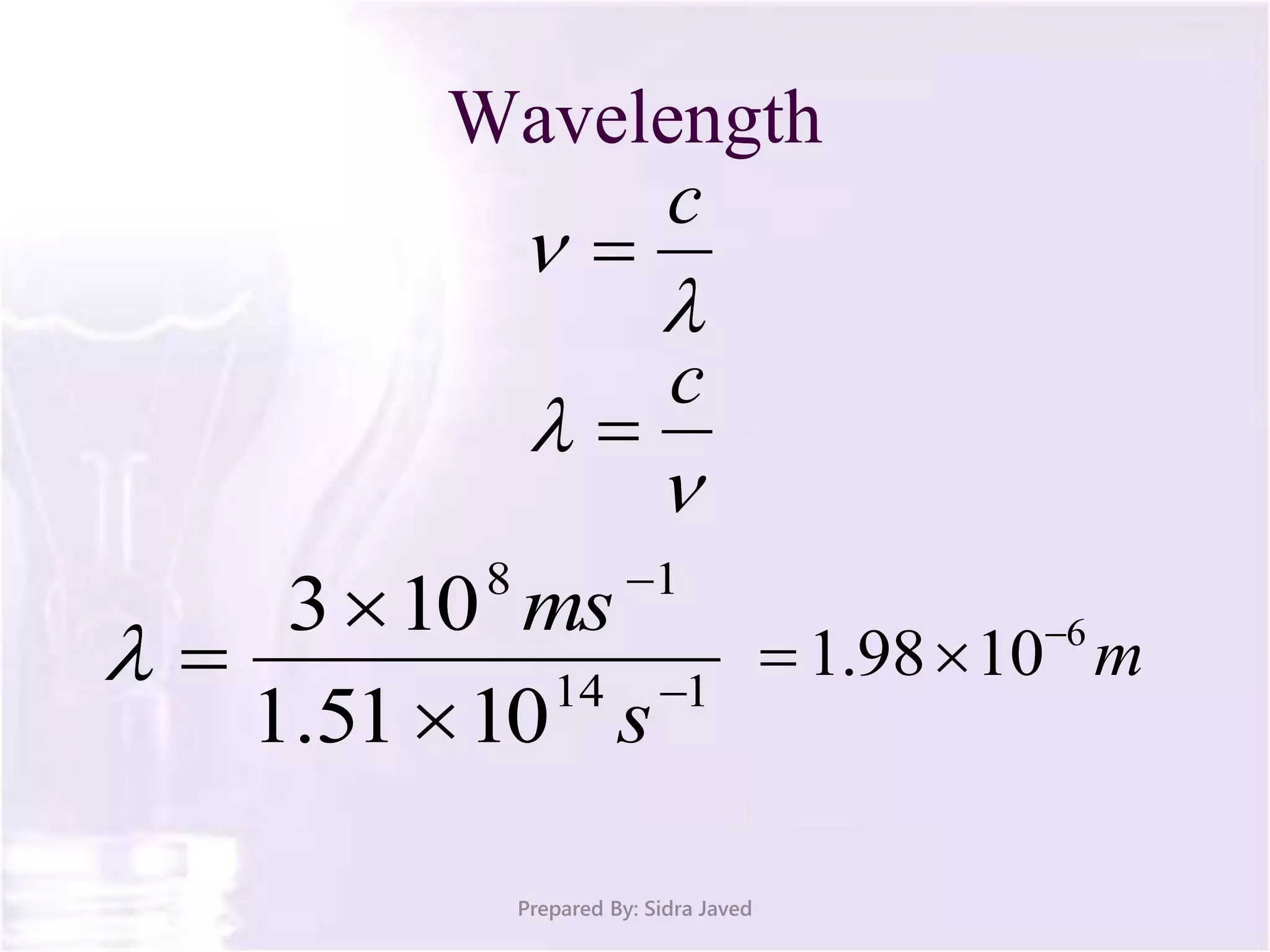
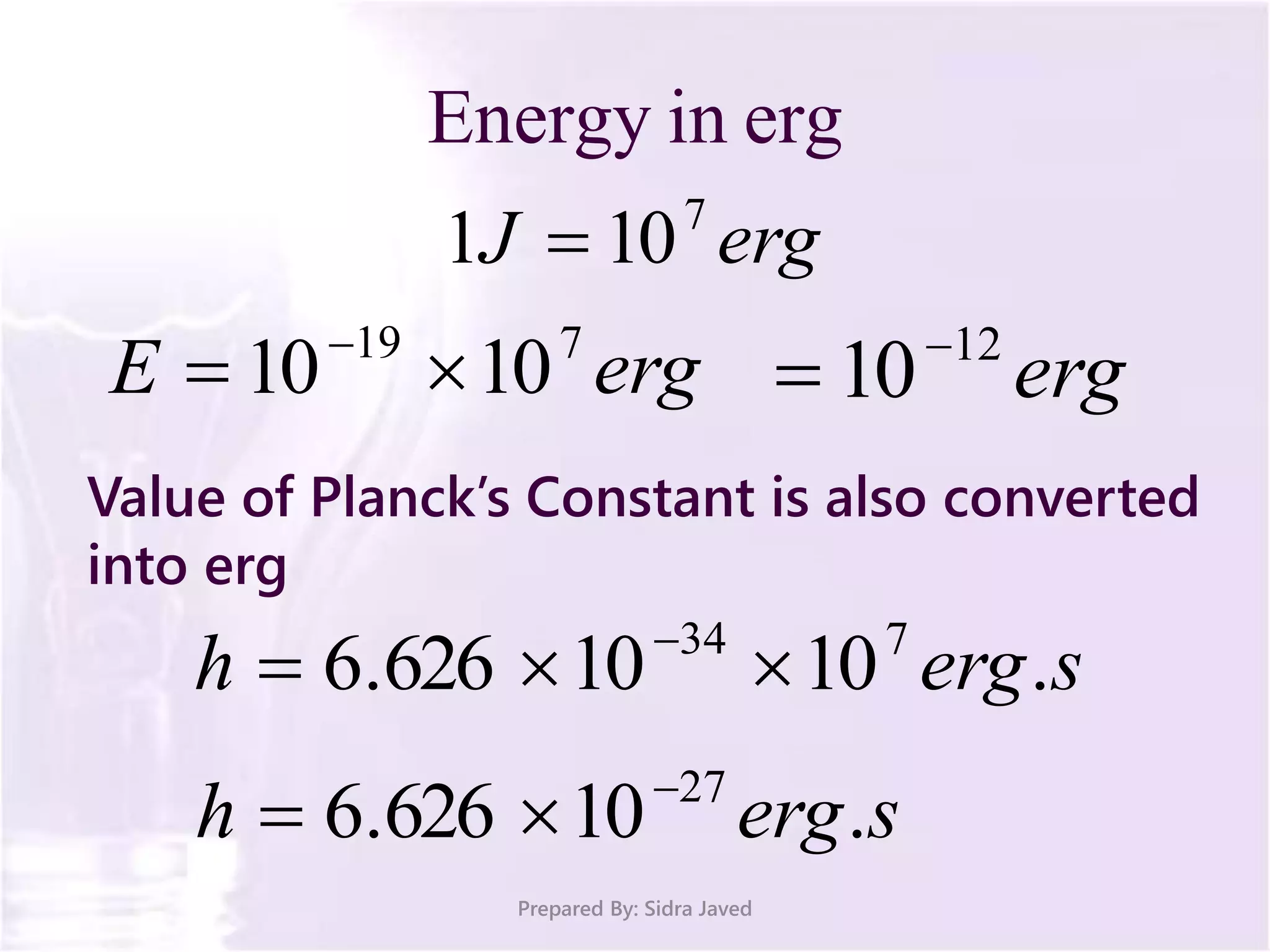
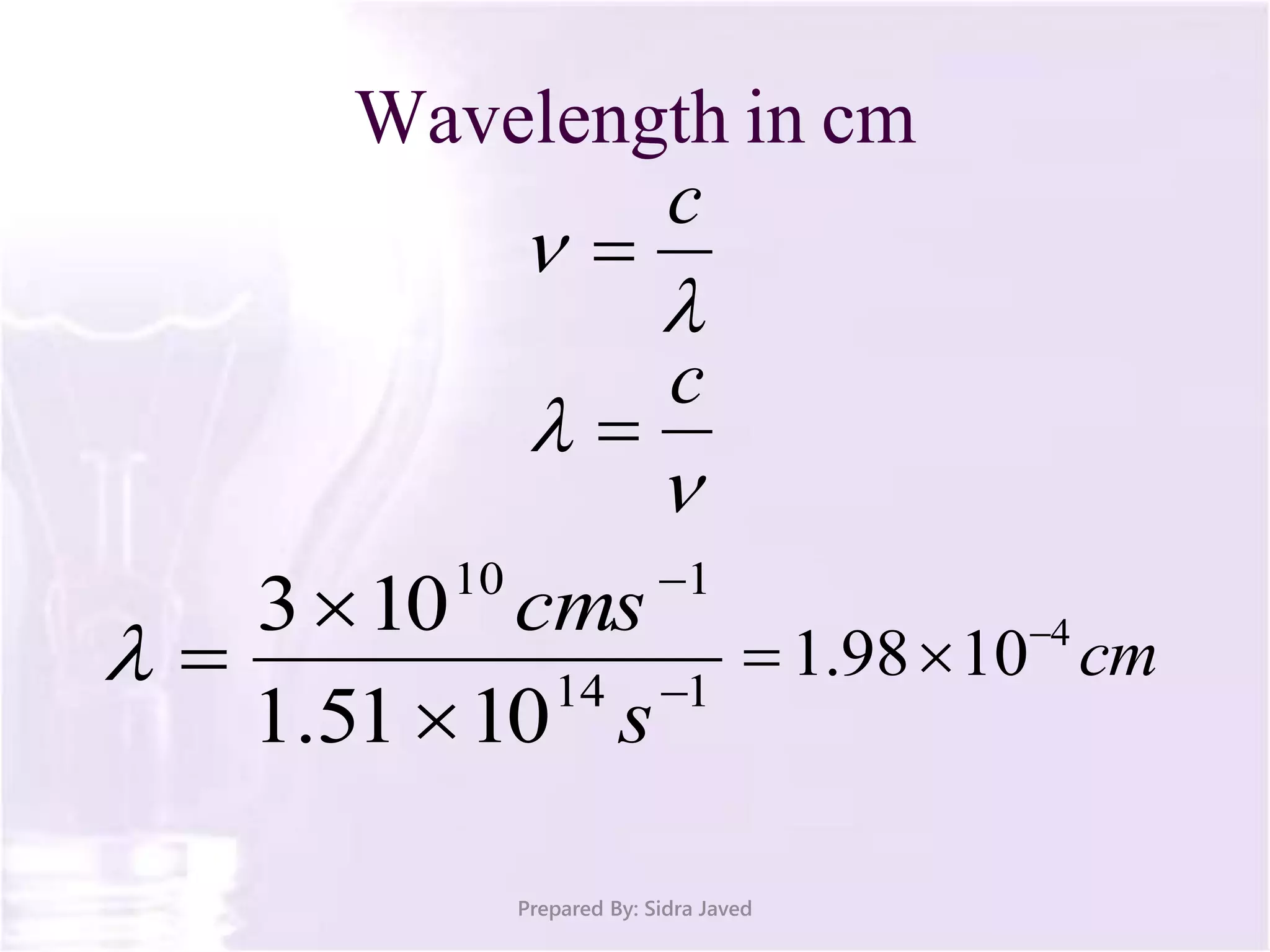
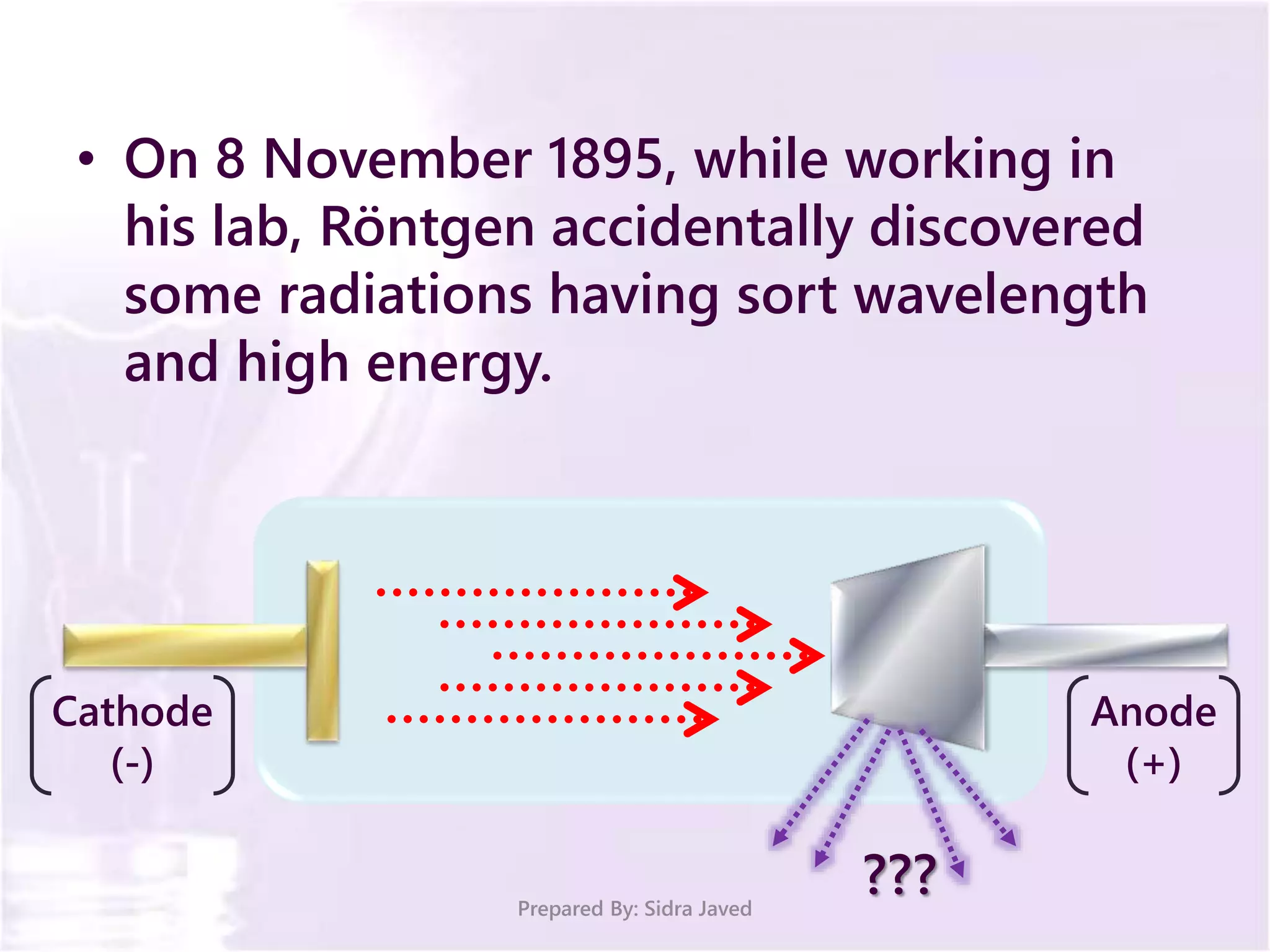
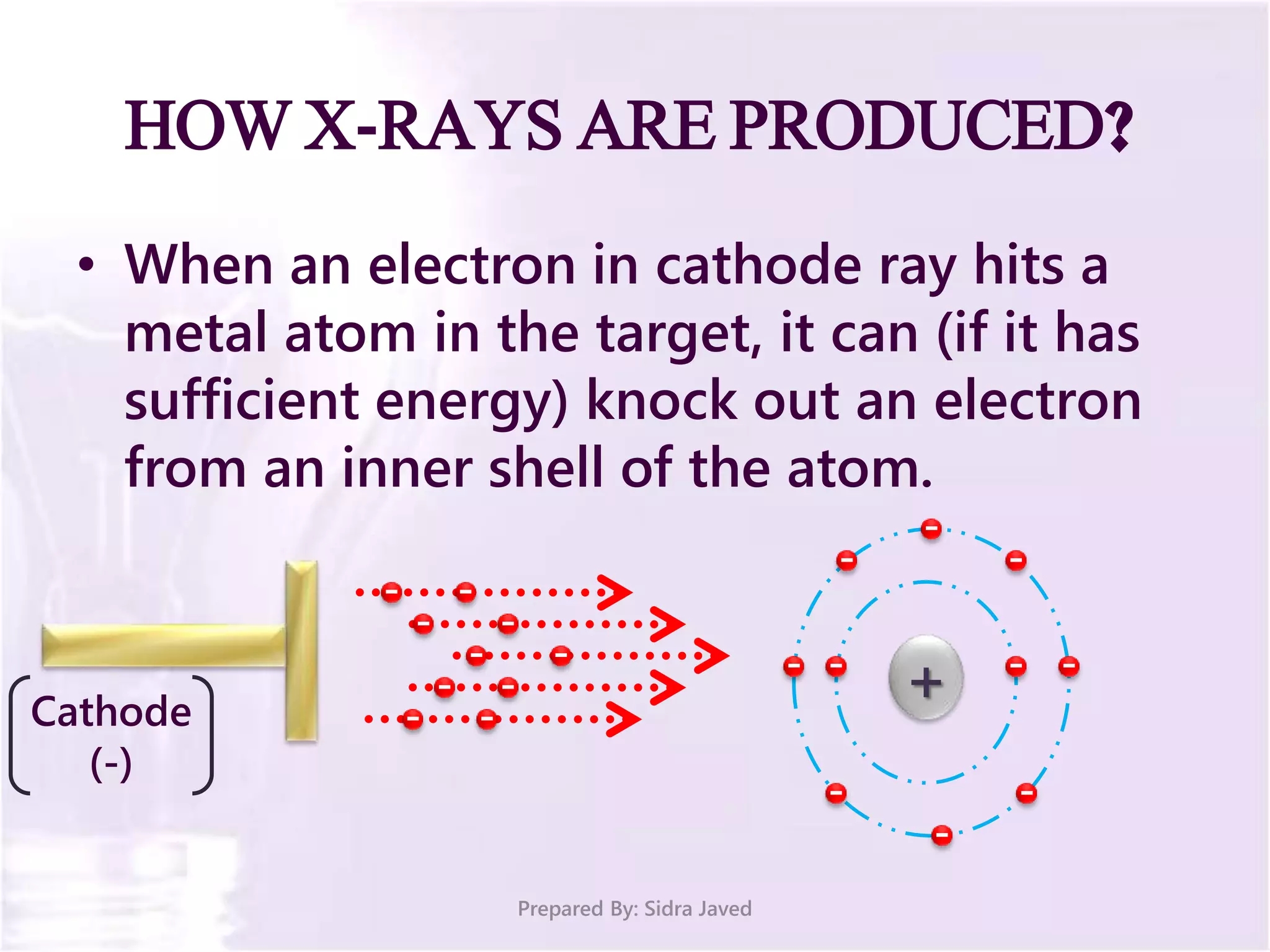
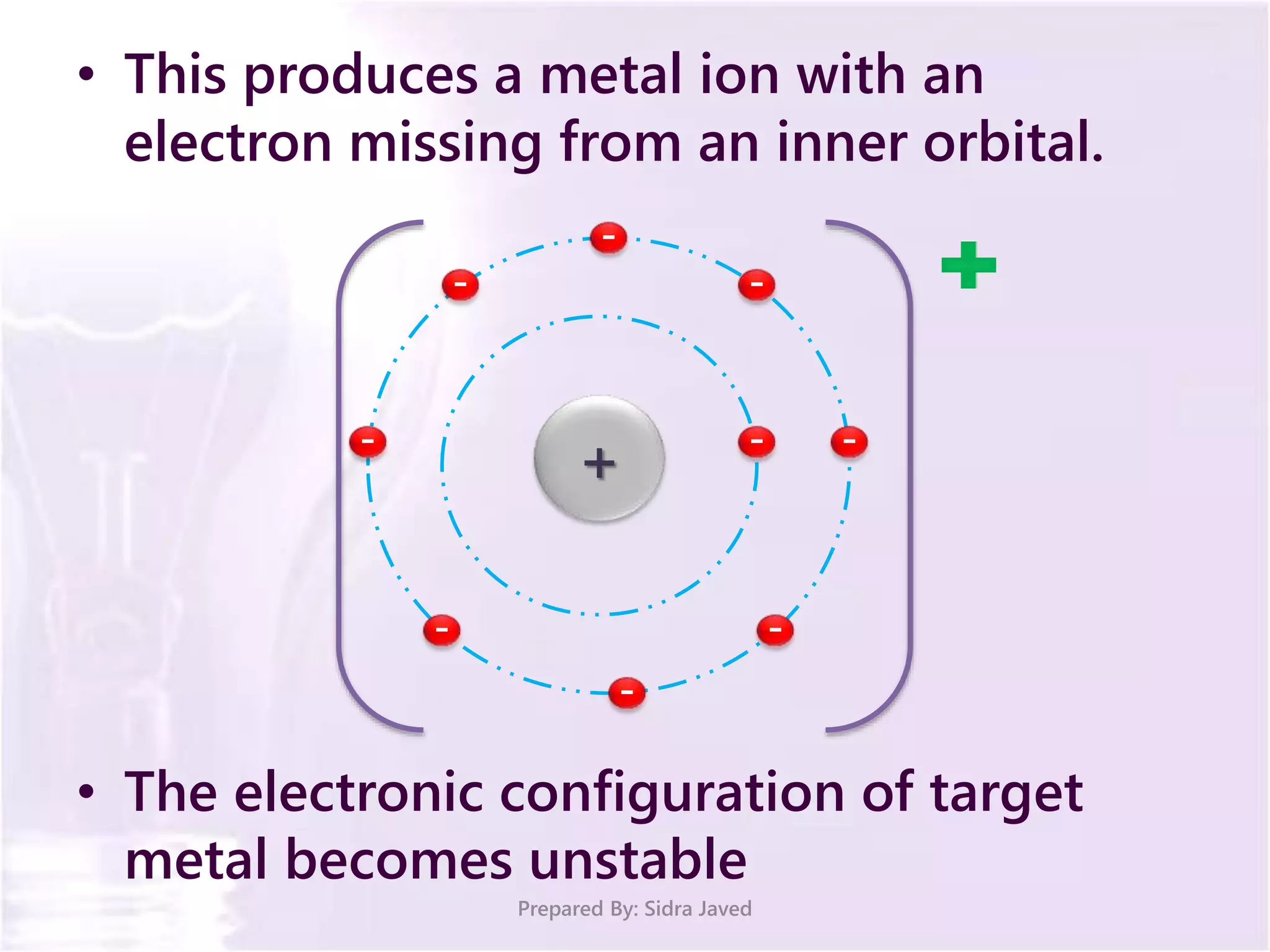
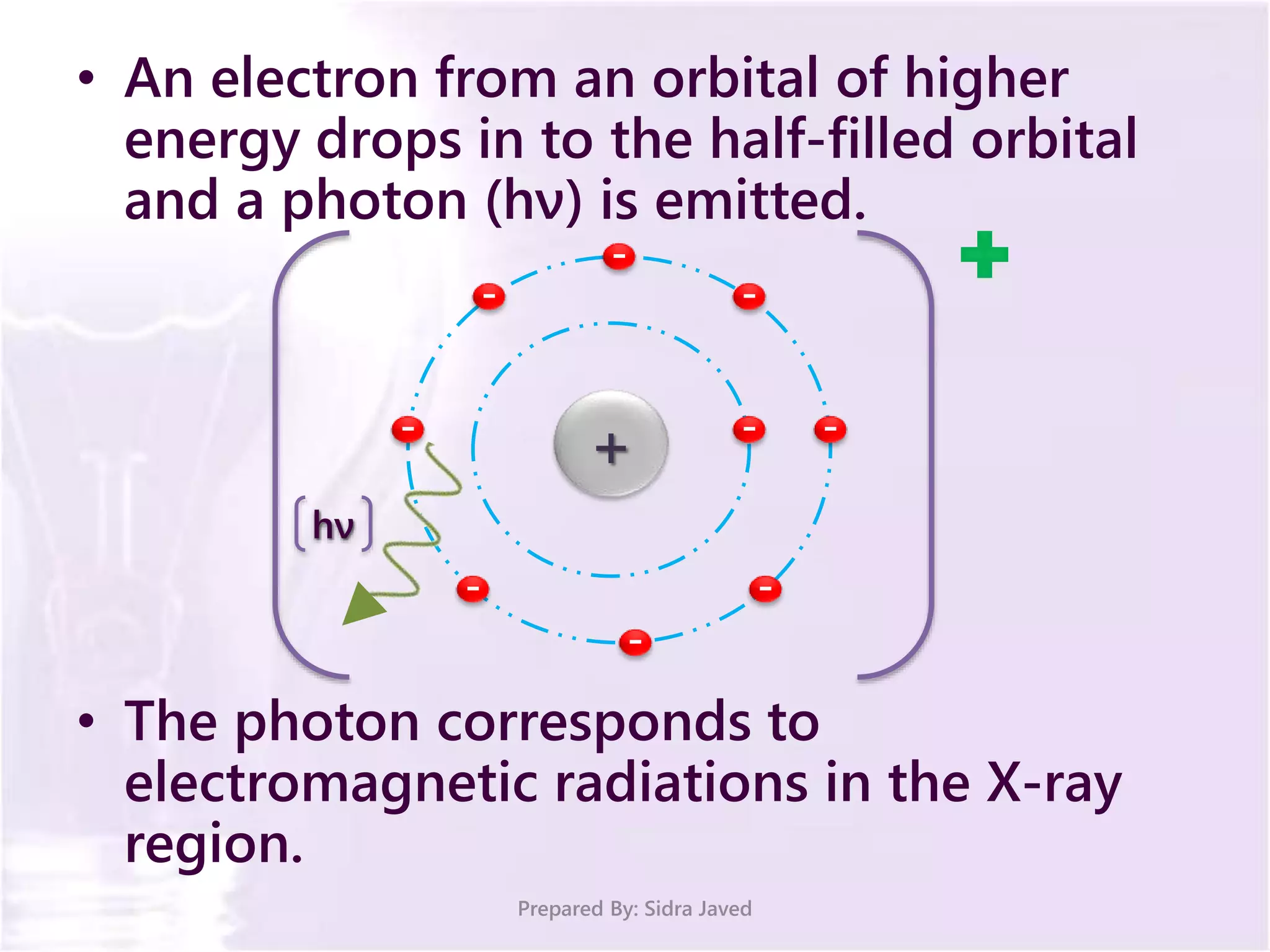
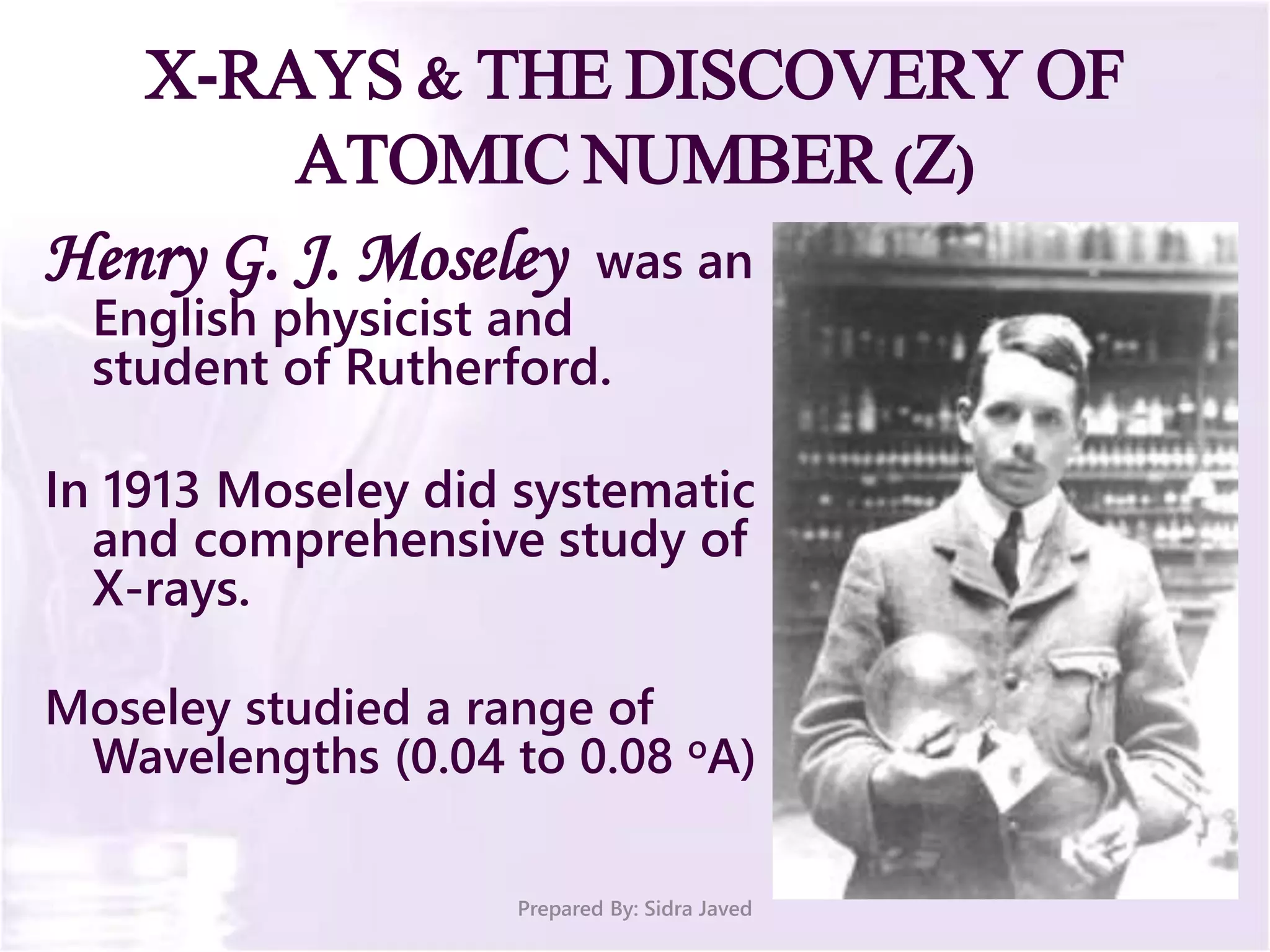
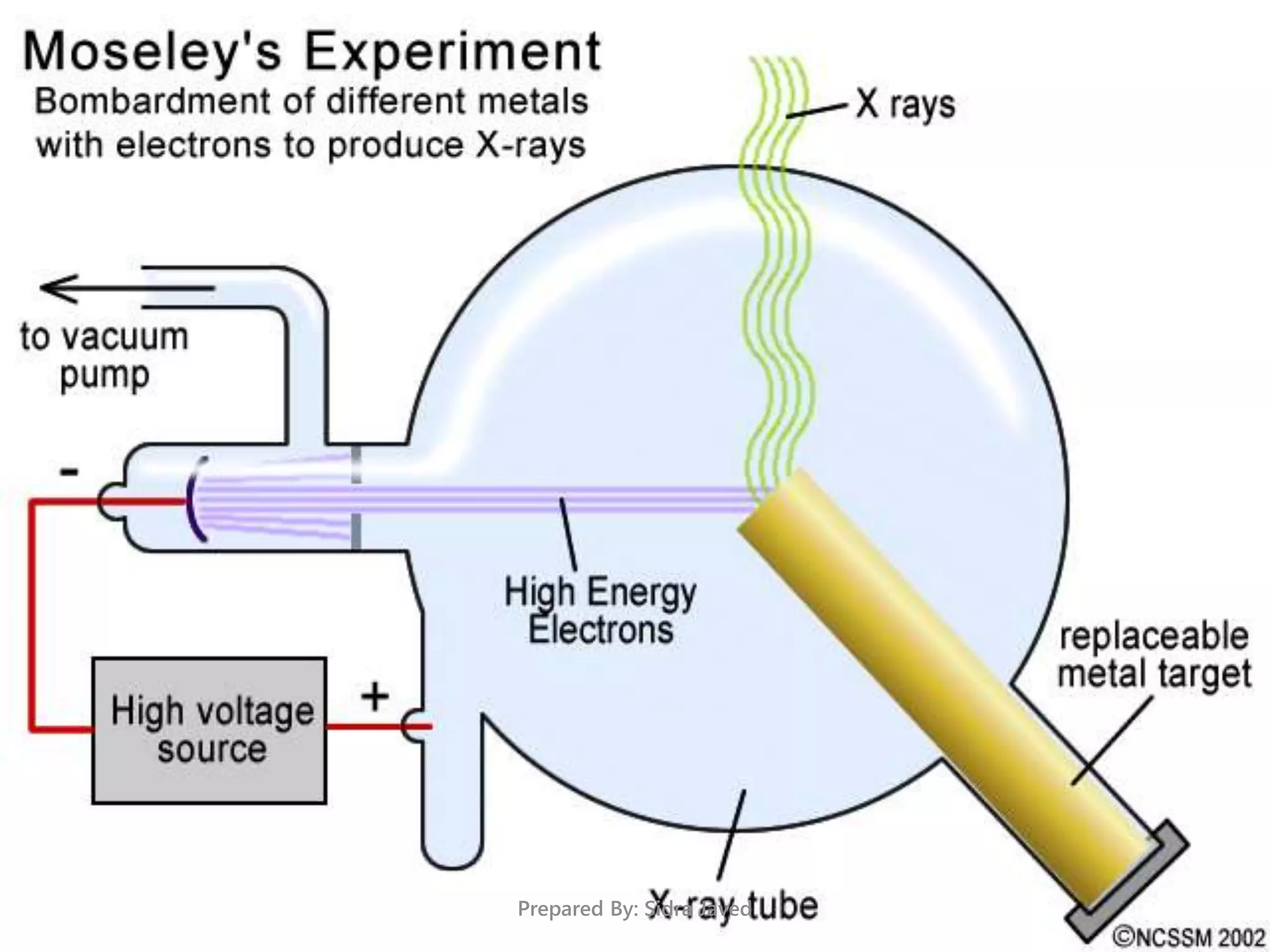
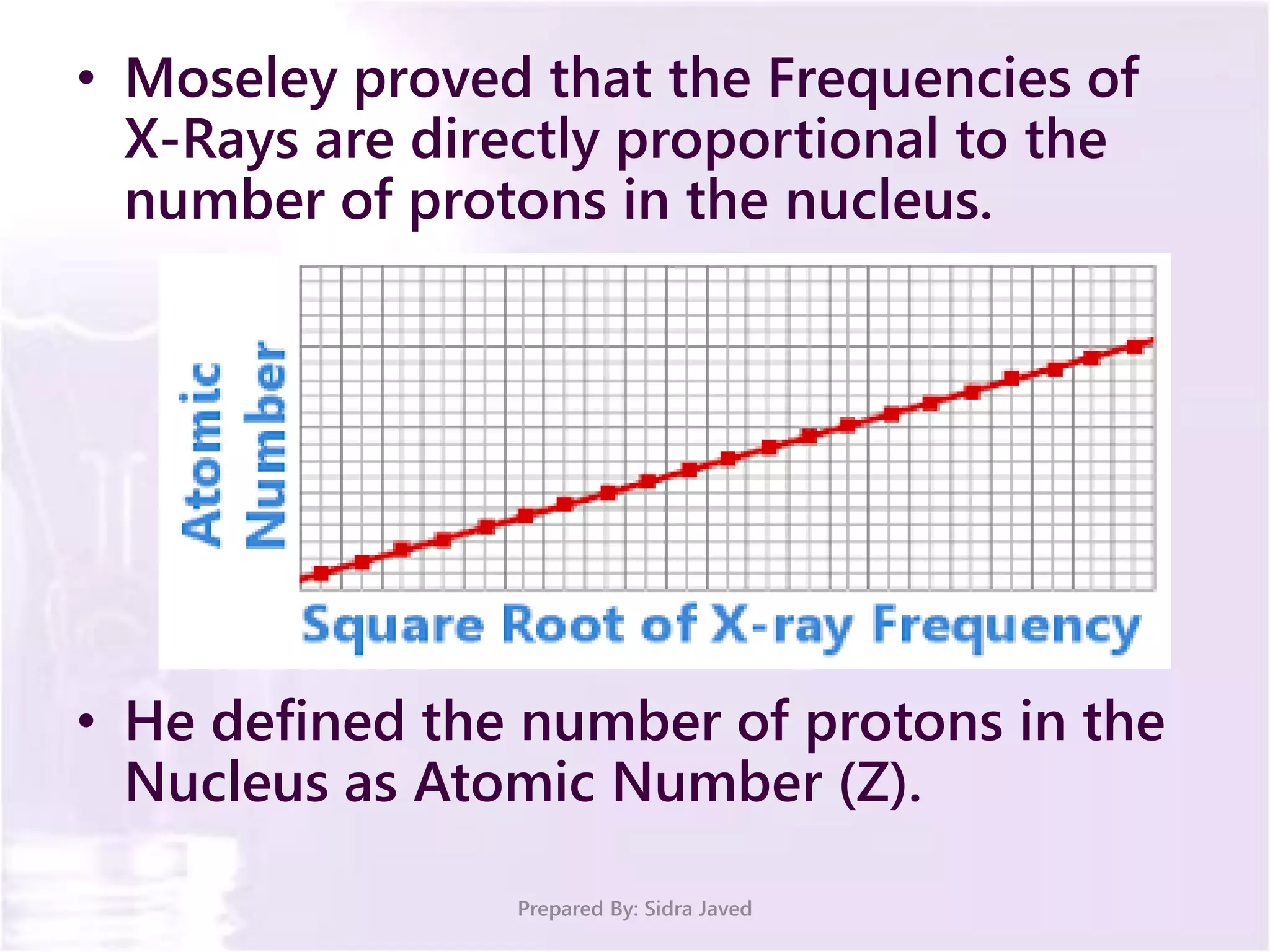
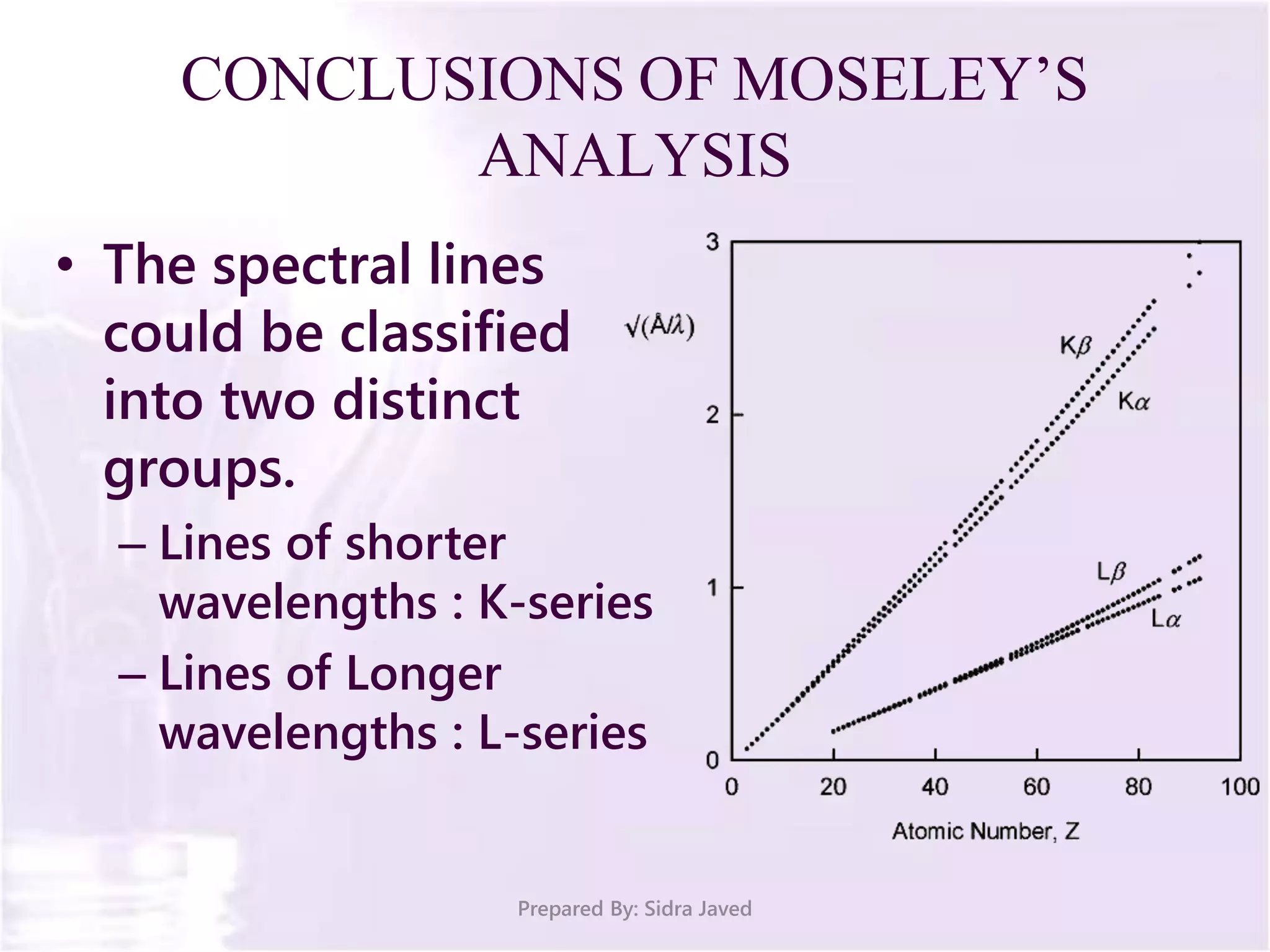
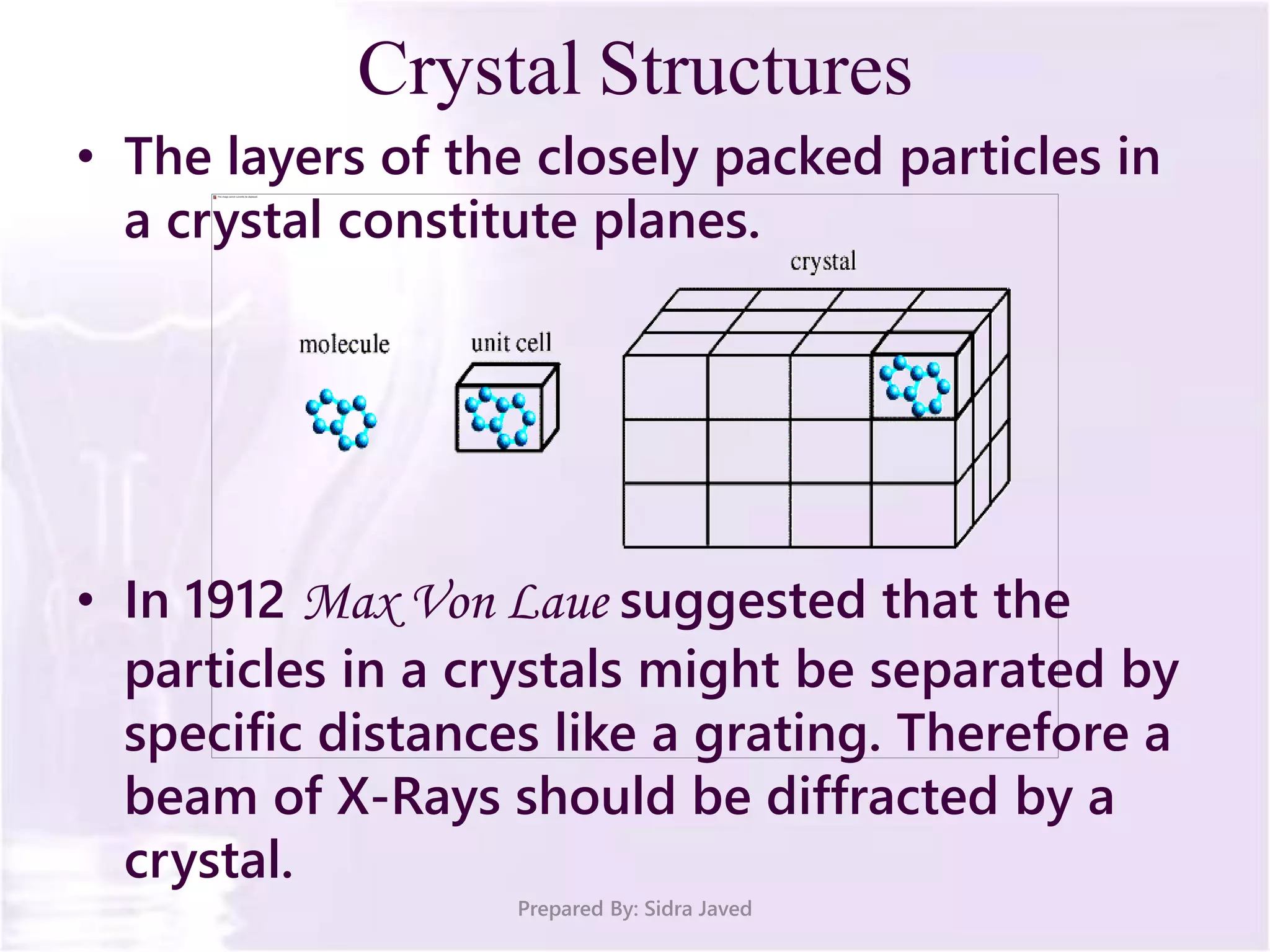
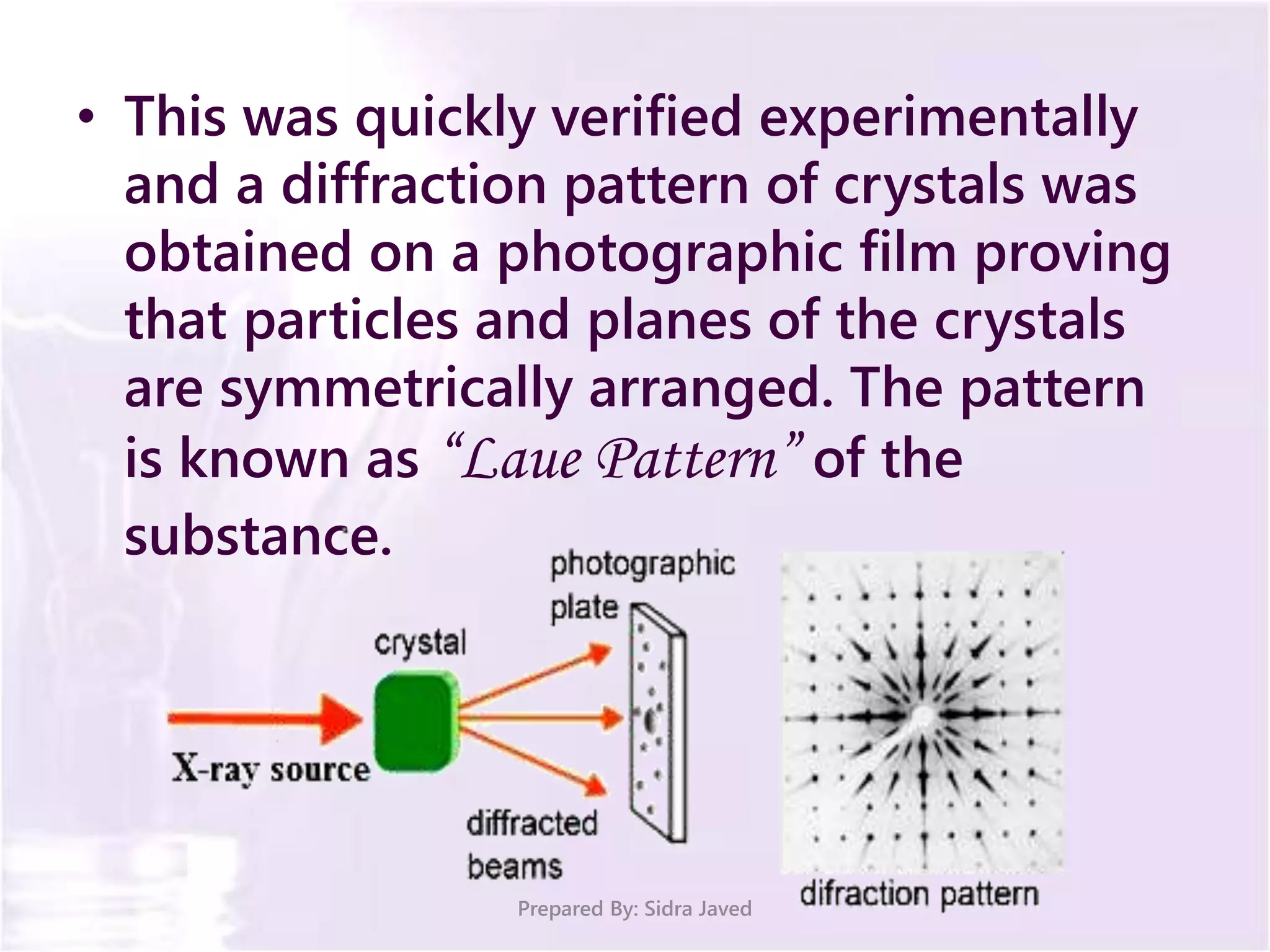
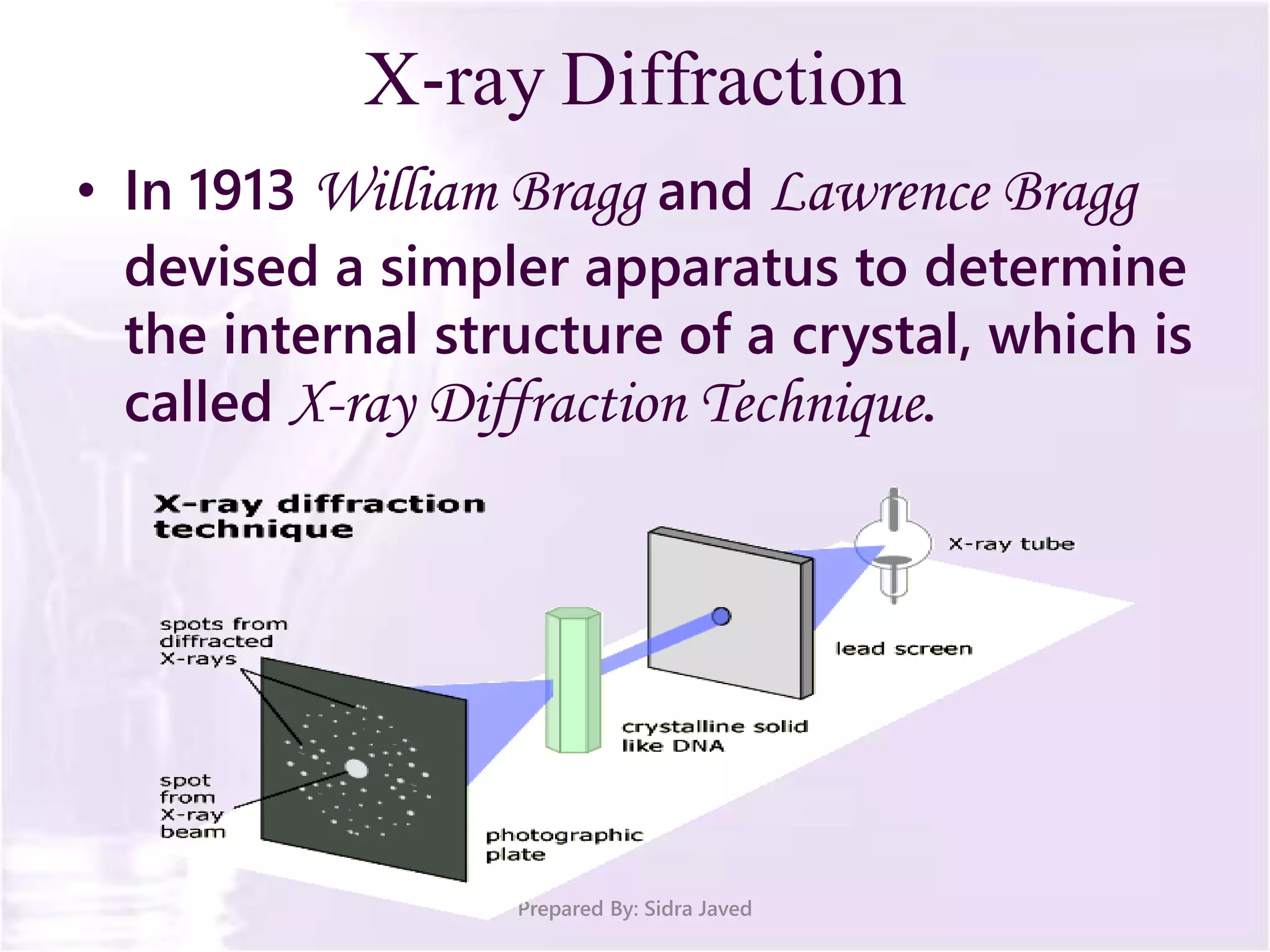
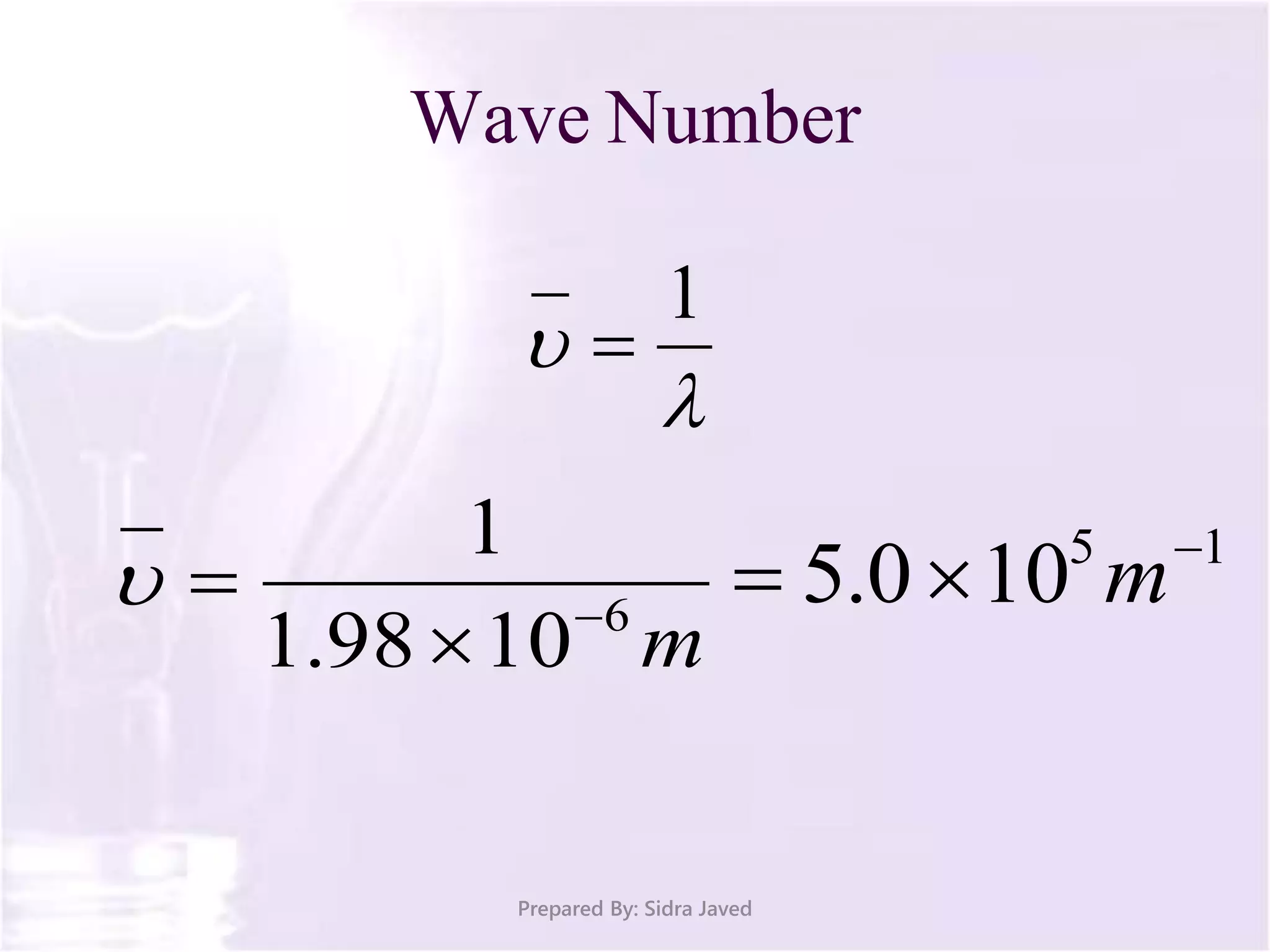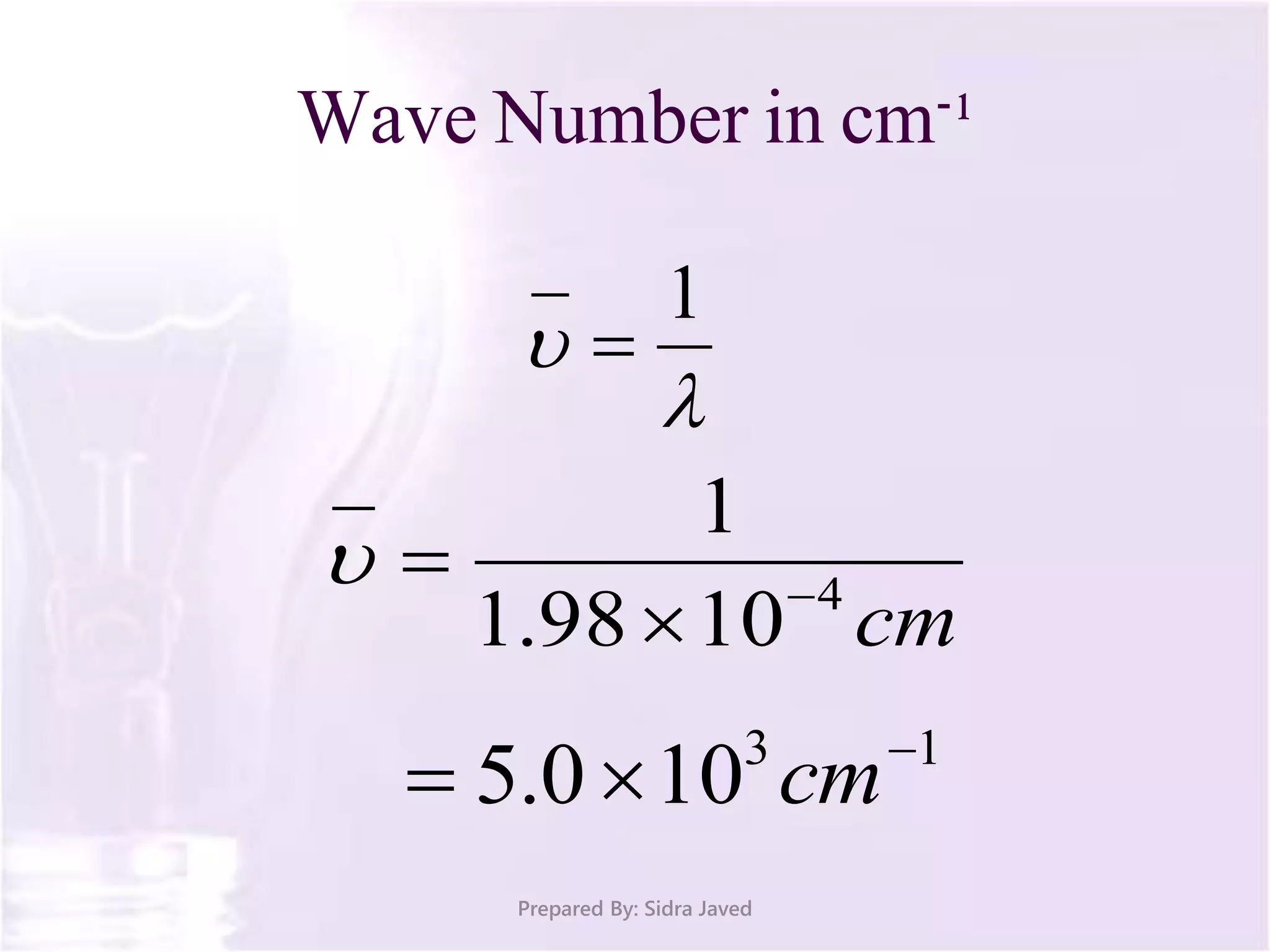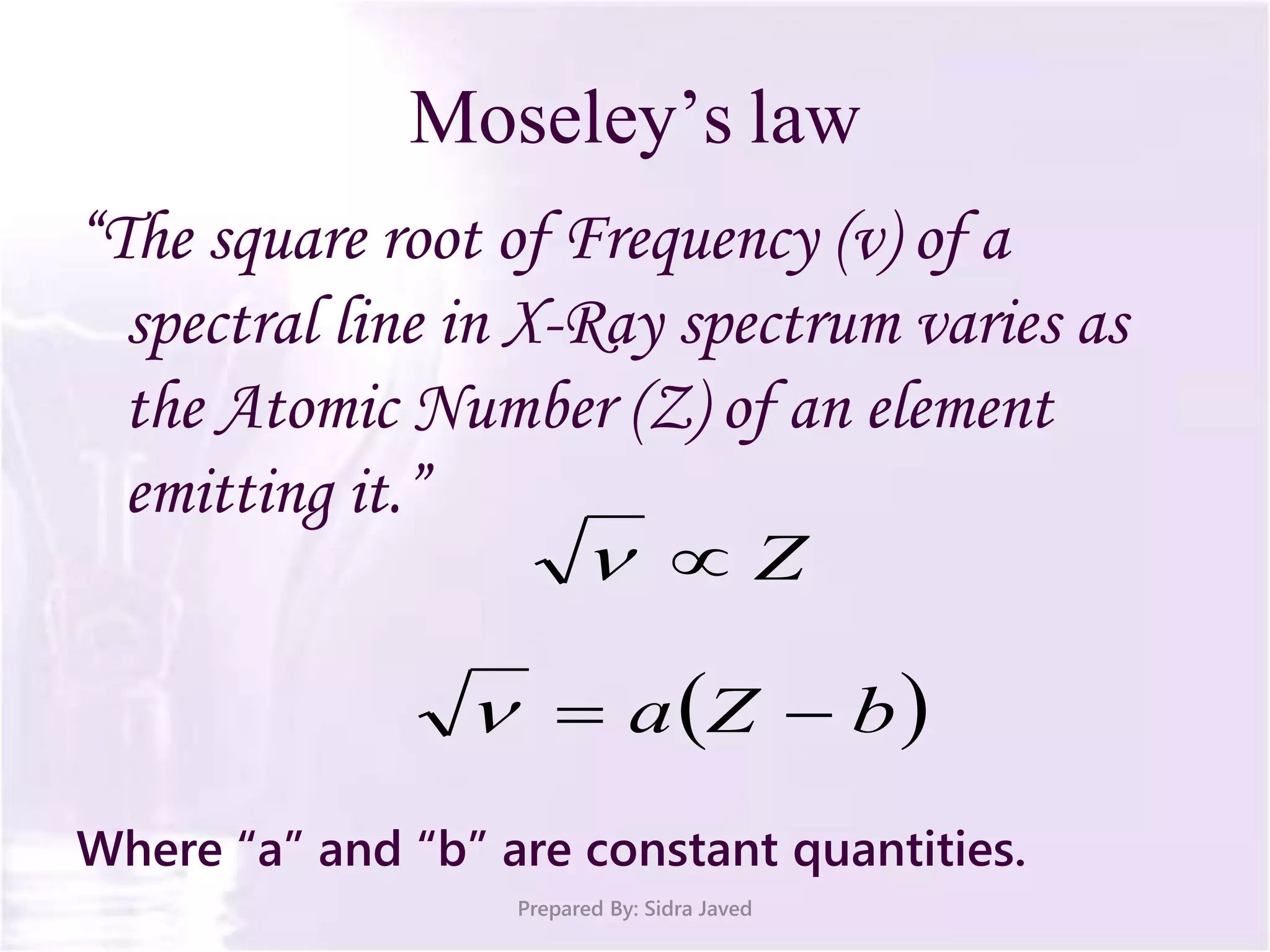The document discusses Planck's quantum theory, including the concept that energy is emitted in discrete quanta or photons, and that the energy is proportional to the frequency of radiation. It also describes the discovery of X-rays by Wilhelm Röntgen and how Henry Moseley's research utilized X-rays to define atomic numbers, leading to Moseley's law linking frequency to atomic number. Furthermore, the document touches on the practical applications of X-rays in medicine and crystallography.